Synapses with short-term plasticity are optimal estimators of presynaptic membrane potentials
- PMID: 20852625
- PMCID: PMC3558743
- DOI: 10.1038/nn.2640
Synapses with short-term plasticity are optimal estimators of presynaptic membrane potentials
Abstract
The trajectory of the somatic membrane potential of a cortical neuron exactly reflects the computations performed on its afferent inputs. However, the spikes of such a neuron are a very low-dimensional and discrete projection of this continually evolving signal. We explored the possibility that the neuron's efferent synapses perform the critical computational step of estimating the membrane potential trajectory from the spikes. We found that short-term changes in synaptic efficacy can be interpreted as implementing an optimal estimator of this trajectory. Short-term depression arose when presynaptic spiking was sufficiently intense as to reduce the uncertainty associated with the estimate; short-term facilitation reflected structural features of the statistics of the presynaptic neuron such as up and down states. Our analysis provides a unifying account of a powerful, but puzzling, form of plasticity.
Figures
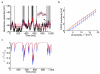
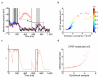
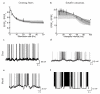

References
-
- Zucker RS, Regehr W. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. - PubMed
-
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. - PubMed
-
- Pfister JP, Dayan P, Lengyel M. Know thy neighbour: A normative theory of synaptic depression. In: Bengio Y, Schuurmans D, Lafferty J, Williams CKI, Culotta A, editors. Advances in Neural Information Processing Systems 22. 2009. pp. 1464–1472.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

