Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism
- PMID: 20852626
- PMCID: PMC3260554
- DOI: 10.1038/nn.2632
Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism
Abstract
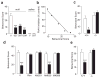
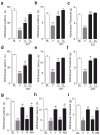
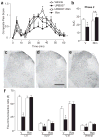
Peripheral cannabinoid receptors exert a powerful inhibitory control over pain initiation, but the endocannabinoid signal that normally engages this intrinsic analgesic mechanism is unknown. To address this question, we developed a peripherally restricted inhibitor (URB937) of fatty acid amide hydrolase (FAAH), the enzyme responsible for the degradation of the endocannabinoid anandamide. URB937 suppressed FAAH activity and increased anandamide levels outside the rodent CNS. Despite its inability to access brain and spinal cord, URB937 attenuated behavioral responses indicative of persistent pain in rodent models of peripheral nerve injury and inflammation and prevented noxious stimulus-evoked neuronal activation in spinal cord regions implicated in nociceptive processing. CB₁ cannabinoid receptor blockade prevented these effects. These results suggest that anandamide-mediated signaling at peripheral CB₁ receptors controls the access of pain-related inputs to the CNS. Brain-impenetrant FAAH inhibitors, which strengthen this gating mechanism, might offer a new approach to pain therapy.
Conflict of interest statement
We wish to acknowledge a conflict of interest. A patent covering URB937 and allied compounds has been filed on behalf of the inventors by the University of California, Irvine, the Italian Institute of Technology, and the Universities of Urbino and Parma.
Figures

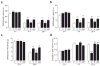
Comment in
-
Endocannabinoids rein in pain outside the brain.Nat Neurosci. 2010 Oct;13(10):1155-6. doi: 10.1038/nn1010-1155. Nat Neurosci. 2010. PMID: 20877276 Free PMC article.
-
Analgesia: Pain control at the periphery.Nat Rev Neurosci. 2010 Nov;11(11):732. doi: 10.1038/nrn2939. Nat Rev Neurosci. 2010. PMID: 20979322
-
Analgesics: Pain control at the periphery.Nat Rev Drug Discov. 2010 Nov;9(11):839. doi: 10.1038/nrd3300. Epub 2010 Oct 29. Nat Rev Drug Discov. 2010. PMID: 21030996 No abstract available.
Similar articles
-
Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment.Pharmacol Res. 2013 Jan;67(1):94-109. doi: 10.1016/j.phrs.2012.10.013. Epub 2012 Nov 2. Pharmacol Res. 2013. PMID: 23127915 Free PMC article.
-
Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain.J Pharmacol Exp Ther. 2009 Sep;330(3):902-10. doi: 10.1124/jpet.109.155465. Epub 2009 Jun 5. J Pharmacol Exp Ther. 2009. PMID: 19502530 Free PMC article.
-
A pro-nociceptive phenotype unmasked in mice lacking fatty-acid amide hydrolase.Mol Pain. 2016 May 13;12:1744806916649192. doi: 10.1177/1744806916649192. Print 2016. Mol Pain. 2016. PMID: 27178246 Free PMC article.
-
Oxidation of the endogenous cannabinoid arachidonoyl ethanolamide by the cytochrome P450 monooxygenases: physiological and pharmacological implications.Pharmacol Rev. 2010 Mar;62(1):136-54. doi: 10.1124/pr.109.001081. Epub 2010 Feb 4. Pharmacol Rev. 2010. PMID: 20133390 Free PMC article. Review.
-
The endocannabinoid system and pain.CNS Neurol Disord Drug Targets. 2009 Dec;8(6):403-21. doi: 10.2174/187152709789824660. CNS Neurol Disord Drug Targets. 2009. PMID: 19839937 Free PMC article. Review.
Cited by
-
The monoacylglycerol lipase inhibitor JZL184 suppresses inflammatory pain in the mouse carrageenan model.Life Sci. 2013 Mar 19;92(8-9):498-505. doi: 10.1016/j.lfs.2012.06.020. Epub 2012 Jun 28. Life Sci. 2013. PMID: 22749865 Free PMC article.
-
A binding site for nonsteroidal anti-inflammatory drugs in fatty acid amide hydrolase.J Am Chem Soc. 2013 Jan 9;135(1):22-5. doi: 10.1021/ja308733u. Epub 2012 Dec 21. J Am Chem Soc. 2013. PMID: 23240907 Free PMC article.
-
Pharmacological characterization of the peripheral FAAH inhibitor URB937 in female rodents: interaction with the Abcg2 transporter in the blood-placenta barrier.Br J Pharmacol. 2012 Dec;167(8):1620-8. doi: 10.1111/j.1476-5381.2012.02098.x. Br J Pharmacol. 2012. PMID: 22774772 Free PMC article.
-
Brain-derived neurotrophic factor and exercise in fibromyalgia syndrome patients: a mini review.Rheumatol Int. 2012 Sep;32(9):2593-9. doi: 10.1007/s00296-011-2348-2. Epub 2011 Dec 31. Rheumatol Int. 2012. PMID: 22210272 Review.
-
Front and hind paw differential analgesic effects of amitriptyline, gabapentin, ibuprofen, and URB937 on mechanical and cold sensitivity in cisplatin-induced neuropathy.Mol Pain. 2019 Jan-Dec;15:1744806919874192. doi: 10.1177/1744806919874192. Mol Pain. 2019. PMID: 31418316 Free PMC article.
References
-
- Stein C, Schafer M, Machelska H. Attacking pain at its source: new perspectives on opioids. Nat Med. 2003;9 (8):1003–1008. - PubMed
-
- Stein C, Zollner C. Opioids and sensory nerves. Handb Exp Pharmacol. 2009;(194):495–518. - PubMed
-
- Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394 (6690):277–281. - PubMed
-
- Jaggar SI, Sellaturay S, Rice AS. The endogenous cannabinoid anandamide, but not the CB2 ligand palmitoylethanolamide, prevents the viscero-visceral hyper-reflexia associated with inflammation of the rat urinary bladder. Neurosci Lett. 1998;253 (2):123–126. - PubMed
-
- Nackley AG, Suplita RL, 2nd, Hohmann AG. A peripheral cannabinoid mechanism suppresses spinal fos protein expression pain behavior in a rat model of inflammation. Neuroscience. 2003;117 (3):659–670. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

