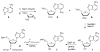Structure and Base Pairing Properties of a Replicable Nonpolar Isostere for Deoxyadenosine
- PMID: 20852720
- PMCID: PMC2939748
- DOI: 10.1021/jo9805100
Structure and Base Pairing Properties of a Replicable Nonpolar Isostere for Deoxyadenosine
Abstract
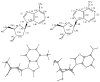
We report the synthesis, structure, and pairing properties in DNA of an isostere for deoxyadenosine which lacks all hydrogen-bonding functionality on the Watson-Crick pairing edge. A deoxyribo-nucleoside derivative of 4-methylbenzimidazole (1), which was recently shown to be inserted into DNA by Klenow DNA polymerase (Morales, J. C.; Kool, E. T. Nature Struct. Biol.1998, 5, 950), is prepared from 1-chloro-2-deoxy-3,5-bis-O-p-toluoyl-α-D-erythro-pentofuranose. The X-ray crystal structure of the nucleoside confirms that the compound is a close steric match for deoxyadenosine (2), although the methylbenzimidazole base is in the syn glycosidic orientation in the crystal. In D(2)O solution, 1H NMR studies show that 1 and 2 have similar (60% vs 70% S) sugar conformations and anti glycosidic orientations. Compound 1 is incorporated into a 12mer oligodeoxynucleotide and its base pairing properties in duplexes assessed by thermal denaturation. The results show that 1 has low affinity for the four natural bases but displays a stronger preference for being situated opposite a nonpolar difluorotoluene nucleoside analogue of thymine (3). The structural similarities of 1 and 2, combined with recent polymerase studies, add support to the hypothesis that steric complementarity plays an important role in base pair replication by polymerase enzymes and that Watson-Crick hydrogen bonds are not absolute requirements. Compound 1 should have significant utility as a probe of the importance of electrostatic effects in protein-DNA and protein-nucleotide binding as well as in DNA replication.
Figures
References
-
- Cristalli G, Franchetti P, Grifantini M, Vittori S, Bordoni T, Geroni C. J Med Chem. 1987;30:1686. - PubMed
-
- Robins RK, Townsend LB, Rousseau RJ. Biochemistry. 1966;5:756. - PubMed
- Ono A, Ueda T. Nucleic Acids Res. 1987;15:3059. - PMC - PubMed
- Cosstick R, Li X, Tuli DK, Williams DM, Connolly BA, Newman PC. Nucleic Acids Res. 1990;18:4771. - PMC - PubMed
- Lever C, Li X, Cosstick R, Ebel S, Brown T. Nucleic Acids Res. 1993;21:1743. - PMC - PubMed
-
- Jones JW, Robins RK. J Am Chem Soc. 1963;85:193.
-
- Nair V, Chamberlain SD. Synthesis. 1984:401.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous