Expression of pax6 and sox2 in adult olfactory epithelium
- PMID: 20852734
- PMCID: PMC2940252
- DOI: 10.1002/cne.22463
Expression of pax6 and sox2 in adult olfactory epithelium
Abstract
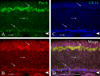
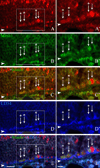
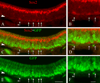
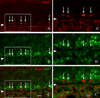
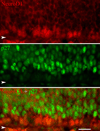
The olfactory epithelium maintains stem and progenitor cells that support the neuroepithelium's life-long capacity to reconstitute after injury. However, the identity of the stem cells--and their regulation--remain poorly defined. The transcription factors Pax6 and Sox2 are characteristic of stem cells in many tissues, including the brain. Therefore, we assessed the expression of Pax6 and Sox2 in normal olfactory epithelium and during epithelial regeneration after methyl bromide lesion or olfactory bulbectomy. Sox2 is found in multiple kinds of cells in normal epithelium, including sustentacular cells, horizontal basal cells, and some globose basal cells. Pax6 is co-expressed with Sox2 in all these, but is also found in duct/gland cells as well as olfactory neurons that innervate necklace glomeruli. Most of the Sox2/Pax6-positive globose basal cells are actively cycling, but some express the cyclin-dependent kinase inhibitor p27(Kip1), and are presumably mitotically quiescent. Among globose basal cells, Sox2 and Pax6 are co-expressed by putatively multipotent progenitors (labeled by neither anti-Mash1 nor anti-Neurog1) and neuron-committed transit amplifying cells (which express Mash1). However, Sox2 and Pax6 are expressed by only a minority of immediate neuronal precursors (Neurog1- and NeuroD1-expressing). The assignment of Sox2 and Pax6 to these categories of globose basal cells is confirmed by a temporal analysis of transcription factor expression during the recovery of the epithelium from methyl bromide-induced injury. Each of the Sox2/Pax6-colabeled cell types is at a remove from the birth of neurons; thus, suppressing their differentiation may be among the roles of Sox2/Pax6 in the olfactory epithelium.
Keywords: neural injury; neural regeneration; tissue stem cells; transcription factors.
Figures









Similar articles
-
Sox2 and Pax6 Play Counteracting Roles in Regulating Neurogenesis within the Murine Olfactory Epithelium.PLoS One. 2016 May 12;11(5):e0155167. doi: 10.1371/journal.pone.0155167. eCollection 2016. PLoS One. 2016. PMID: 27171428 Free PMC article.
-
Ascl1 (Mash1) knockout perturbs differentiation of nonneuronal cells in olfactory epithelium.PLoS One. 2012;7(12):e51737. doi: 10.1371/journal.pone.0051737. Epub 2012 Dec 17. PLoS One. 2012. PMID: 23284756 Free PMC article.
-
Combinatorial regulation of optic cup progenitor cell fate by SOX2 and PAX6.Development. 2011 Feb;138(3):443-54. doi: 10.1242/dev.055178. Development. 2011. PMID: 21205789 Free PMC article.
-
Role of a transcription factor Pax6 in the developing vertebrate olfactory system.Dev Growth Differ. 2007 Dec;49(9):683-90. doi: 10.1111/j.1440-169X.2007.00965.x. Epub 2007 Oct 1. Dev Growth Differ. 2007. PMID: 17908181 Review.
-
Stem and progenitor cells of the mammalian olfactory epithelium: Taking poietic license.J Comp Neurol. 2017 Mar 1;525(4):1034-1054. doi: 10.1002/cne.24105. Epub 2016 Sep 27. J Comp Neurol. 2017. PMID: 27560601 Free PMC article. Review.
Cited by
-
Disparate progenitor cell populations contribute to maintenance and repair neurogenesis in the zebrafish olfactory epithelium.Cell Tissue Res. 2022 May;388(2):331-358. doi: 10.1007/s00441-022-03597-x. Epub 2022 Mar 10. Cell Tissue Res. 2022. PMID: 35266039
-
Purinergic Signaling Pathway in Human Olfactory Neuronal Precursor Cells.Stem Cells Int. 2019 Apr 2;2019:2728786. doi: 10.1155/2019/2728786. eCollection 2019. Stem Cells Int. 2019. PMID: 31065271 Free PMC article.
-
Adult c-Kit(+) progenitor cells are necessary for maintenance and regeneration of olfactory neurons.J Comp Neurol. 2015 Jan 1;523(1):15-31. doi: 10.1002/cne.23653. Epub 2014 Aug 25. J Comp Neurol. 2015. PMID: 25044230 Free PMC article.
-
Following the p63/Keratin5 basal cells in the sensory and non-sensory epithelia of the vomeronasal organ.Genesis. 2024 Apr;62(2):e23596. doi: 10.1002/dvg.23596. Genesis. 2024. PMID: 38665067 Free PMC article.
-
Neural crest and olfactory system: new prospective.Mol Neurobiol. 2012 Oct;46(2):349-60. doi: 10.1007/s12035-012-8286-5. Epub 2012 Jul 8. Mol Neurobiol. 2012. PMID: 22773137 Free PMC article. Review.
References
-
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94(2):239–247. - PubMed
-
- Bani-Yaghoub M, Tremblay RG, Lei JX, Zhang D, Zurakowski B, Sandhu JK, Smith B, Ribecco-Lutkiewicz M, Kennedy J, Walker PR, Sikorska M. Role of Sox2 in the development of the mouse neocortex. Dev Biol. 2006;295(1):52–66. - PubMed
-
- Blake JA, Thomas M, Thompson JA, White R, Ziman M. Perplexing Pax: from puzzle to paradigm. Dev Dyn. 2008;237(10):2791–2803. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

