Imaging cells in three-dimensional collagen matrix
- PMID: 20853341
- PMCID: PMC2988473
- DOI: 10.1002/0471143030.cb1018s48
Imaging cells in three-dimensional collagen matrix
Abstract
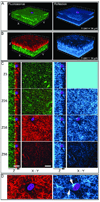
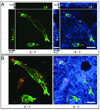
The use of in vitro three-dimensional (3-D) collagen matrices to mimic an in vivo cellular environment has become increasingly popular and is broadening our understanding of cellular processes and cell-ECM interactions. To study cells in in vitro 3-D collagen matrices, both cellular proteins and the collagen matrix must be visualized. In this unit, the authors describe the protocol and provide troubleshooting for immunolabeling of cells in 3-D collagen gels to localize and visualize cellular proteins with high-resolution fluorescence confocal microscopy. The authors then describe confocal reflection microscopy as a technique for direct imaging of 3-D fibrillar collagen matrices by discussing the advantages and disadvantages of the technique. They also provide instrument settings required for simultaneous imaging of cellular proteins with fluorescence confocal imaging and 3-D collagen fibrils with confocal reflection microscopy. Additionally, the authors provide protocols for a "cell sandwiching" technique to prepare cell cultures in 3-D collagen matrices required for high-resolution confocal imaging.
© 2010 by John Wiley & Sons, Inc.
Figures


Similar articles
-
Dynamic three-dimensional visualization of collagen matrix remodeling and cytoskeletal organization in living corneal fibroblasts.Scanning. 2004 Jan-Feb;26(1):1-10. doi: 10.1002/sca.4950260102. Scanning. 2004. PMID: 15000286
-
Differential interference contrast and confocal reflectance imaging of collagen organization in three-dimensional matrices.Scanning. 2006 Nov-Dec;28(6):305-10. doi: 10.1002/sca.4950280602. Scanning. 2006. PMID: 17181131
-
Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure.J Biomech Eng. 2002 Apr;124(2):214-22. doi: 10.1115/1.1449904. J Biomech Eng. 2002. PMID: 12002131
-
Techniques for assessing 3-D cell-matrix mechanical interactions in vitro and in vivo.Exp Cell Res. 2013 Oct 1;319(16):2470-80. doi: 10.1016/j.yexcr.2013.06.018. Epub 2013 Jun 29. Exp Cell Res. 2013. PMID: 23819988 Free PMC article. Review.
-
Toward single cell traction microscopy within 3D collagen matrices.Exp Cell Res. 2013 Oct 1;319(16):2396-408. doi: 10.1016/j.yexcr.2013.06.009. Epub 2013 Jun 25. Exp Cell Res. 2013. PMID: 23806281 Free PMC article. Review.
Cited by
-
Targeted gene silencing of CCL2 inhibits triple negative breast cancer progression by blocking cancer stem cell renewal and M2 macrophage recruitment.Oncotarget. 2016 Aug 2;7(31):49349-49367. doi: 10.18632/oncotarget.9885. Oncotarget. 2016. PMID: 27283985 Free PMC article.
-
Experimental and computational analyses reveal that environmental restrictions shape HIV-1 spread in 3D cultures.Nat Commun. 2019 May 13;10(1):2144. doi: 10.1038/s41467-019-09879-3. Nat Commun. 2019. PMID: 31086185 Free PMC article.
-
Phototunable interpenetrating polymer network hydrogels to stimulate the vasculogenesis of stem cell-derived endothelial progenitors.Acta Biomater. 2021 Mar 1;122:133-144. doi: 10.1016/j.actbio.2020.12.041. Epub 2020 Dec 21. Acta Biomater. 2021. PMID: 33359297 Free PMC article.
-
Discoidin domain receptor 2 regulates aberrant mesenchymal lineage cell fate and matrix organization.Sci Adv. 2022 Dec 21;8(51):eabq6152. doi: 10.1126/sciadv.abq6152. Epub 2022 Dec 21. Sci Adv. 2022. PMID: 36542719 Free PMC article.
-
Revealing the cytoskeletal organization of invasive cancer cells in 3D.J Vis Exp. 2013 Oct 26;(80):e50763. doi: 10.3791/50763. J Vis Exp. 2013. PMID: 24192916 Free PMC article.
References
-
- Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17(5):524–532. - PubMed
-
- Eyre DR, Paz MA, et al. Cross-linking in collagen and elastin. Annu Rev Biochem. 1984;53:717–748. - PubMed
-
- Gelman RA, Williams BR, et al. Collagen fibril formation. Evidence for a multistep process. J Biol Chem. 1979;254(1):180–186. - PubMed
-
- Pedersen JA, Swartz MA. Mechanobiology in the third dimension. Ann Biomed Eng. 2005;33(11):1469–1490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

