Tuberoinfundibular peptide of 39 residues modulates the mouse hypothalamic-pituitary-adrenal axis via paraventricular glutamatergic neurons
- PMID: 20853513
- PMCID: PMC3004125
- DOI: 10.1002/cne.22462
Tuberoinfundibular peptide of 39 residues modulates the mouse hypothalamic-pituitary-adrenal axis via paraventricular glutamatergic neurons
Abstract
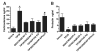
Neurons in the subparafascicular area at the caudal border of the thalamus that contain the neuropeptide tuberoinfundibular peptide of 39 residues (TIP39) densely innervate several hypothalamic areas, including the paraventricular nucleus (PVN). These areas contain a matching distribution of TIP39's receptor, the parathyroid hormone receptor 2 (PTH2R). Frequent PTH2R coexpression with a vesicular glutamate transporter (VGlut2) suggests that TIP39 could presynaptically regulate glutamate release. By using immunohistochemistry we found CRH-ir neurons surrounded by PTH2R-ir fibers and TIP39-ir axonal projections in the PVN area of the mouse brain. Labeling hypothalamic neuroendocrine neurons by peripheral injection of fluorogold in PTH2R-lacZ knock-in mice showed that most PTH2Rs are on PVN and peri-PVN interneurons and not on neuroendocrine cells. Double fluorescent in situ hybridization revealed a high level of coexpression between PTH2R and VGlut2 mRNA by cells located in the PVN and nearby brain areas. Local TIP39 infusion (100 pmol) robustly increased pCREB-ir in the PVN and adjacent perinuclear zone. It also increased plasma corticosterone and decreased plasma prolactin. These effects of TIP39 on pCREB-ir, corticosterone, and prolactin were abolished by coinfusion of the ionotropic glutamate receptor antagonists 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and DL-2-amino-5-phosphonopentanoic acid (AP-5; 30 pmol each) and were absent in PTH2R knockout mice. Basal plasma corticosterone was slightly decreased in TIP39 knockout mice just before onset of their active phase. The present data indicate that the TIP39 ligand/PTH2 receptor system provides facilitatory regulation of the hypothalamic-pituitary-adrenal axis via hypothalamic glutamatergic neurons and that it may regulate other neuroendocrine systems by a similar mechanism.
Published 2010 Wiley-Liss, Inc.
Figures







Similar articles
-
Tuberoinfundibular peptide of 39 residues (TIP39) signaling modulates acute and tonic nociception.Exp Neurol. 2010 Nov;226(1):68-83. doi: 10.1016/j.expneurol.2010.08.004. Epub 2010 Aug 6. Exp Neurol. 2010. PMID: 20696160 Free PMC article.
-
Parathyroid hormone 2 receptor and its endogenous ligand tuberoinfundibular peptide of 39 residues are concentrated in endocrine, viscerosensory and auditory brain regions in macaque and human.Neuroscience. 2009 Aug 4;162(1):128-47. doi: 10.1016/j.neuroscience.2009.04.054. Epub 2009 May 3. Neuroscience. 2009. PMID: 19401215 Free PMC article.
-
Regulation of hypothalamic signaling by tuberoinfundibular peptide of 39 residues is critical for the response to cold: a novel peptidergic mechanism of thermoregulation.J Neurosci. 2011 Dec 7;31(49):18166-79. doi: 10.1523/JNEUROSCI.2619-11.2011. J Neurosci. 2011. PMID: 22159128 Free PMC article.
-
The TIP39-PTH2 receptor system: unique peptidergic cell groups in the brainstem and their interactions with central regulatory mechanisms.Prog Neurobiol. 2010 Jan 11;90(1):29-59. doi: 10.1016/j.pneurobio.2009.10.017. Epub 2009 Oct 24. Prog Neurobiol. 2010. PMID: 19857544 Free PMC article. Review.
-
Emerging functions for tuberoinfundibular peptide of 39 residues.Trends Endocrinol Metab. 2003 Jan;14(1):14-9. doi: 10.1016/s1043-2760(02)00002-4. Trends Endocrinol Metab. 2003. PMID: 12475607 Review.
Cited by
-
Behavioural actions of tuberoinfundibular peptide 39 (parathyroid hormone 2).J Neuroendocrinol. 2022 Sep;34(9):e13130. doi: 10.1111/jne.13130. Epub 2022 May 2. J Neuroendocrinol. 2022. PMID: 35499975 Free PMC article. Review.
-
Tuberoinfundibular peptide of 39 residues (TIP39) signaling modulates acute and tonic nociception.Exp Neurol. 2010 Nov;226(1):68-83. doi: 10.1016/j.expneurol.2010.08.004. Epub 2010 Aug 6. Exp Neurol. 2010. PMID: 20696160 Free PMC article.
-
Molecular and functional diversity of GABA-A receptors in the enteric nervous system of the mouse colon.J Neurosci. 2014 Jul 30;34(31):10361-78. doi: 10.1523/JNEUROSCI.0441-14.2014. J Neurosci. 2014. PMID: 25080596 Free PMC article.
-
Conserved multisensory integration of social cues in the thalamus.iScience. 2024 Dec 22;28(1):111678. doi: 10.1016/j.isci.2024.111678. eCollection 2025 Jan 17. iScience. 2024. PMID: 39868040 Free PMC article. Review.
-
Persistent histamine excitation of glutamatergic preoptic neurons.PLoS One. 2012;7(10):e47700. doi: 10.1371/journal.pone.0047700. Epub 2012 Oct 17. PLoS One. 2012. PMID: 23082195 Free PMC article.
References
-
- Bjorklund A, Nobin A. Fluorescence histochemical and microspectrofluorometric mapping of dopamine and noradrena-line cell groups in the rat diencephalon. Brain Res. 1973;51:193–205. - PubMed
-
- Bregonzio C, Navarro CE, Donoso AO. NMDA receptor antagonists block stress-induced prolactin release in female rats at estrus. Eur J Pharmacol. 1998;350:259–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

