SDF-1α secreted by human CD133-derived multipotent stromal cells promotes neural progenitor cell survival through CXCR7
- PMID: 20854168
- PMCID: PMC3102777
- DOI: 10.1089/scd.2010.0198
SDF-1α secreted by human CD133-derived multipotent stromal cells promotes neural progenitor cell survival through CXCR7
Abstract
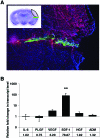
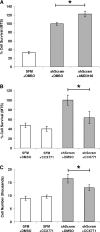
We recently reported that concentrated conditioned medium (CdM) from human CD133-derived bone marrow progenitor cells (CD133 CdM) was neuroprotective after stroke. Here we identify stromal-derived factor 1 alpha (SDF-1) as a potential neuroprotective candidate in CD133 CdM by interrogating the transcriptional responses of CD133-derived multipotent stromal cells (CD133dMSCs) after cell injection into the ischemic brain. Human SDF-1 mRNA was upregulated 79-fold by CD133dMSCs when injected into the stroke peri-infarct area compared with cells injected into the uninjured parenchyma of sham-operated animals. In cell protection assays, we replaced the typical growth medium in mouse neural progenitor cell (mNPC) cultures with serum-free CD133 CdM immediately before exposure to hypoxia (1% oxygen) for 48 h. CD133 CdM significantly increased the survival of mNPCs during hypoxia exposure and growth factor withdrawal. To determine whether MSC-secreted SDF-1 influenced mNPC survival, we used lentiviral short hairpin RNA against SDF1 (shSDF-1) to knockdown SDF-1 expression in CD133dMSCs. The CdM generated from shSDF-1-treated cells had a 94% decrease in secreted SDF-1 and was significantly less protective for mNPCs when compared with control CdM from CD133dMSCs transduced with scrambled short hairpin RNA. Pharmacological inhibition of the 2 known SDF-1 receptors, CXCR4 and CXCR7, revealed that only CXCR7 activity was functionally linked to survival signaling in mNPCs during hypoxia exposure. Treatment of mNPCs with CD133 CdM and CXCR7 inhibitor decreased mNPC viability by 36.5% ± 12.8% and decreased cell number by 21% ± 6.7% compared with dimethyl sulfoxide treated controls. These data indicate that SDF-1 is a key neuroprotective cytokine secreted by CD133dMSCs that protects mNPCs through CXCR7.
Figures


References
-
- Kinnaird T. Stabile E. Burnett MS. Shou M. Lee CW. Barr S. Fuchs S. Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. - PubMed
-
- Kurozumi K. Nakamura K. Tamiya T. Kawano Y. Ishii K. Kobune M. Hirai S. Uchida H. Sasaki K. Ito Y. Kato K. Honmou O. Houkin K. Date I. Hamada H. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther. 2005;11:96–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

