The multiple facets of HIV attachment to dendritic cell lectins
- PMID: 20854332
- PMCID: PMC7162262
- DOI: 10.1111/j.1462-5822.2010.01519.x
The multiple facets of HIV attachment to dendritic cell lectins
Abstract
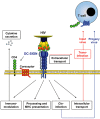
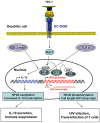
Entry of enveloped viruses into host cells depends on the interactions of viral surface proteins with cell surface receptors. Many enveloped viruses maximize the efficiency of receptor engagement by first binding to attachment-promoting factors, which concentrate virions on target cells and thus increase the likelihood of subsequent receptor engagement. Cellular lectins can recognize glycans on viral surface proteins and mediate viral uptake into immune cells for subsequent antigen presentation. Paradoxically, many viral and non-viral pathogens target lectins to attach to immune cells and to subvert cellular functions to promote their spread. Thus, it has been proposed that attachment of HIV to the dendritic cell lectin DC-SIGN enables the virus to hijack cellular transport processes to ensure its transmission to adjacent T cells. However, recent studies show that the consequences of viral capture by immune cell lectins can be diverse, and can entail negative and positive regulation of viral spread. Here, we will describe key concepts proposed for the role of lectins in HIV attachment to host cells, and we will discuss recent findings in this rapidly evolving area of research.
© 2010 Blackwell Publishing Ltd.
Figures
Similar articles
-
A division of labor: DC subsets and HIV receptor diversity.Nat Immunol. 2002 Oct;3(10):891-3. doi: 10.1038/ni1002-891. Nat Immunol. 2002. PMID: 12352961 No abstract available.
-
HIV-1 N-glycan composition governs a balance between dendritic cell-mediated viral transmission and antigen presentation.J Immunol. 2011 Nov 1;187(9):4676-85. doi: 10.4049/jimmunol.1101876. Epub 2011 Sep 28. J Immunol. 2011. PMID: 21957147 Free PMC article.
-
Receptor usage dictates HIV-1 restriction by human TRIM5α in dendritic cell subsets.Nature. 2016 Dec 15;540(7633):448-452. doi: 10.1038/nature20567. Epub 2016 Dec 7. Nature. 2016. PMID: 27919079
-
Sugar and spice: viral envelope-DC-SIGN interactions in HIV pathogenesis.Curr HIV Res. 2003 Jan;1(1):87-99. doi: 10.2174/1570162033352129. Curr HIV Res. 2003. PMID: 15043214 Review.
-
Distinct roles for DC-SIGN+-dendritic cells and Langerhans cells in HIV-1 transmission.Trends Mol Med. 2008 Jan;14(1):12-9. doi: 10.1016/j.molmed.2007.11.001. Epub 2007 Dec 4. Trends Mol Med. 2008. PMID: 18055263 Review.
Cited by
-
HIV-1 envelope-receptor interactions required for macrophage infection and implications for current HIV-1 cure strategies.J Leukoc Biol. 2014 Jan;95(1):71-81. doi: 10.1189/jlb.0713368. Epub 2013 Oct 24. J Leukoc Biol. 2014. PMID: 24158961 Free PMC article. Review.
-
Filovirus entry: a novelty in the viral fusion world.Viruses. 2012 Feb;4(2):258-75. doi: 10.3390/v4020258. Epub 2012 Feb 7. Viruses. 2012. PMID: 22470835 Free PMC article. Review.
-
Ebola virus outbreak, updates on current therapeutic strategies.Rev Med Virol. 2015 Jul;25(4):241-53. doi: 10.1002/rmv.1841. Epub 2015 May 11. Rev Med Virol. 2015. PMID: 25962887 Free PMC article. Review.
-
Severe fever with thrombocytopenia virus glycoproteins are targeted by neutralizing antibodies and can use DC-SIGN as a receptor for pH-dependent entry into human and animal cell lines.J Virol. 2013 Apr;87(8):4384-94. doi: 10.1128/JVI.02628-12. Epub 2013 Feb 6. J Virol. 2013. PMID: 23388721 Free PMC article.
-
Exclusive Decoration of Simian Immunodeficiency Virus Env with High-Mannose Type N-Glycans Is Not Compatible with Mucosal Transmission in Rhesus Macaques.J Virol. 2015 Nov;89(22):11727-33. doi: 10.1128/JVI.01358-15. Epub 2015 Sep 9. J Virol. 2015. PMID: 26355090 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

