SCFFbx2-E3-ligase-mediated degradation of BACE1 attenuates Alzheimer's disease amyloidosis and improves synaptic function
- PMID: 20854419
- PMCID: PMC3307224
- DOI: 10.1111/j.1474-9726.2010.00632.x
SCFFbx2-E3-ligase-mediated degradation of BACE1 attenuates Alzheimer's disease amyloidosis and improves synaptic function
Abstract
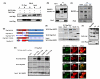
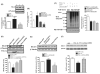
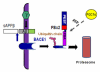
BACE1 (β-secretase) plays a central role in the β-amyloidogenesis of Alzheimer's disease (AD). The ubiquitin-proteasome system, a major intracellular protein quality control system, has been implicated recently in BACE1 metabolism. We report that the SCF(Fbx2) -E3 ligase is involved in the binding and ubiquitination of BACE1 via its Trp 280 residue of F-box-associated domain. Physiologically, we found that Fbx2 was expressed in various intracellular organelles in brain neurons and that BACE1 is colocalized with Fbx2 and the amyloid precursor protein (APP), mainly at the early endosome and endoplasmic reticulum. The former are believed to be the major intracellular compartments where the APP is cleaved by BACE1 and β-amyloid is produced. Importantly, we found that overexpression of Fbx2 in the primary cortical and hippocampal neurons derived from Tg2576 transgenic mice significantly promoted BACE1 degradation and reduced β-amyloid production. In the search for specific endogenous modulators of Fbx2 expression, we found that PPARγ coactivator-1α (PGC-1α) was capable of promoting the degradation of BACE1 through a mechanism involving Fbx2 gene expression. Interestingly, we found that the expression of both Fbx2 and PGC-1α was significantly decreased in the brains of aging Tg2576 mice. Our in vivo studies using a mouse model of AD revealed that exogenous adenoviral Fbx2 expression in the brain significantly decreased BACE1 protein levels and activity, coincidentally reducing β-amyloid levels and rescuing synaptic deficits. Our study is the first to suggest that promoting Fbx2 in the brain may represent a novel strategy for the treatment of AD.
© 2010 The Authors. Aging Cell © 2010 Blackwell Publishing Ltd/Anatomical Society of Great Britain and Ireland.
Figures





Similar articles
-
CHIP stabilizes amyloid precursor protein via proteasomal degradation and p53-mediated trans-repression of β-secretase.Aging Cell. 2015 Aug;14(4):595-604. doi: 10.1111/acel.12335. Epub 2015 Mar 13. Aging Cell. 2015. PMID: 25773675 Free PMC article.
-
Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer's mouse models.Neurobiol Aging. 2013 Jun;34(6):1581-8. doi: 10.1016/j.neurobiolaging.2012.12.005. Epub 2013 Jan 9. Neurobiol Aging. 2013. PMID: 23312803 Free PMC article.
-
Relationship between ubiquilin-1 and BACE1 in human Alzheimer's disease and APdE9 transgenic mouse brain and cell-based models.Neurobiol Dis. 2016 Jan;85:187-205. doi: 10.1016/j.nbd.2015.11.005. Epub 2015 Nov 10. Neurobiol Dis. 2016. PMID: 26563932
-
BACE1: the beta-secretase enzyme in Alzheimer's disease.J Mol Neurosci. 2004;23(1-2):105-14. doi: 10.1385/JMN:23:1-2:105. J Mol Neurosci. 2004. PMID: 15126696 Review.
-
The beta-secretase, BACE: a prime drug target for Alzheimer's disease.J Mol Neurosci. 2001 Oct;17(2):157-70. doi: 10.1385/JMN:17:2:157. J Mol Neurosci. 2001. PMID: 11816789 Review.
Cited by
-
Genetically engineered mouse models for functional studies of SKP1-CUL1-F-box-protein (SCF) E3 ubiquitin ligases.Cell Res. 2013 May;23(5):599-619. doi: 10.1038/cr.2013.44. Epub 2013 Mar 26. Cell Res. 2013. PMID: 23528706 Free PMC article. Review.
-
CHIP stabilizes amyloid precursor protein via proteasomal degradation and p53-mediated trans-repression of β-secretase.Aging Cell. 2015 Aug;14(4):595-604. doi: 10.1111/acel.12335. Epub 2015 Mar 13. Aging Cell. 2015. PMID: 25773675 Free PMC article.
-
Estrogenic Endocrine Disrupting Chemicals Influencing NRF1 Regulated Gene Networks in the Development of Complex Human Brain Diseases.Int J Mol Sci. 2016 Dec 13;17(12):2086. doi: 10.3390/ijms17122086. Int J Mol Sci. 2016. PMID: 27983596 Free PMC article. Review.
-
Relationships between Mitochondrial Dysfunction and Neurotransmission Failure in Alzheimer's Disease.Aging Dis. 2020 Oct 1;11(5):1291-1316. doi: 10.14336/AD.2019.1125. eCollection 2020 Oct. Aging Dis. 2020. PMID: 33014538 Free PMC article. Review.
-
Neuropsychological benefits of stationary bike exercise and a cybercycle exergame for older adults with diabetes: an exploratory analysis.J Diabetes Sci Technol. 2012 Jul 1;6(4):849-57. doi: 10.1177/193229681200600416. J Diabetes Sci Technol. 2012. PMID: 22920811 Free PMC article.
References
-
- Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat. Rev. Mol. Cell Biol. 2004;5:739–751. - PubMed
-
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat. Neurosci. 1999;2:271–276. - PubMed
-
- Ciechanover A, Brundin P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron. 2003;40:427–446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

