S-adenosyl methionine improves early viral responses and interferon-stimulated gene induction in hepatitis C nonresponders
- PMID: 20854821
- PMCID: PMC3021477
- DOI: 10.1053/j.gastro.2010.09.010
S-adenosyl methionine improves early viral responses and interferon-stimulated gene induction in hepatitis C nonresponders
Abstract
Background & aims: Less than half of patients infected with hepatitis C virus (HCV) achieve sustained viral clearance after pegylated interferon (peginterferon) and ribavirin therapy. S-adenosyl methionine (SAMe) improves interferon signaling in cell culture. We assessed the effect of SAMe on the kinetics of the early antiviral response and interferon signaling in nonresponders to previous antiviral therapy and investigated the mechanisms involved.
Methods: Nonresponders with HCV genotype 1 were given peginterferon alfa-2a and ribavirin for 2 weeks (course A, baseline/control). After 1 month, patients received SAMe (1600 mg daily) for 2 weeks and then peginterferon and ribavirin for 48 weeks (course B; completed by 21 of 24 patients). Viral kinetics and interferon-stimulated gene (ISG) expression in peripheral blood mononuclear cells (PBMCs) were compared between courses.
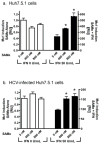
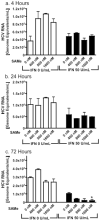
Results: The decrease in HCV RNA from 0 to 48 hours (phase 1) was similar with and without SAMe. However, the second phase slope of viral decline was improved with SAMe (course A, 0.11 ± 0.04 log(10) IU/mL/wk; course B, 0.27 ± 0.06; P = .009); 11 patients (53%) achieved an early virological response, and 10 (48%) had undetectable HCV RNA by week 24. Induction of ISGs in PBMCs was significantly greater during course B. In cultured cells, SAMe increased induction of ISGs and the antiviral effects of interferon by increasing STAT1 methylation, possibly affecting STAT1-DNA binding.
Conclusions: The addition of SAMe to peginterferon and ribavirin improves the early viral kinetics and increases ISG induction in nonresponders to previous therapy. SAMe might be a useful adjunct to peginterferon-based therapies in chronic HCV infection.
Trial registration: ClinicalTrials.gov NCT00475176.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. - PubMed
-
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–65. - PubMed
-
- Camma C, Cabibbo G, Bronte F, Enea M, Licata A, Attanasio M, Andriulli A, Craxi A. Retreatment with pegylated interferon plus ribavirin of chronic hepatitis C non-responders to interferon plus ribavirin: a meta-analysis. J Hepatol. 2009;51:675–81. - PubMed
-
- Jensen DM, Marcellin P, Freilich B, Andreone P, Di Bisceglie A, Brandao-Mello CE, Reddy KR, Craxi A, Martin AO, Teuber G, Messinger D, Thommes JA, Tietz A. Re-treatment of patients with chronic hepatitis C who do not respond to peginterferon-alpha2b: a randomized trial. Ann Intern Med. 2009;150:528–40. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

