Stimulation of α2-adrenergic receptors in the central nucleus of the amygdala attenuates stress-induced reinstatement of nicotine seeking in rats
- PMID: 20854830
- PMCID: PMC3014445
- DOI: 10.1016/j.neuropharm.2010.09.013
Stimulation of α2-adrenergic receptors in the central nucleus of the amygdala attenuates stress-induced reinstatement of nicotine seeking in rats
Abstract
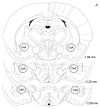
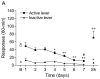
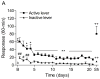
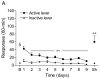
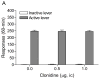
Tobacco addiction is a chronic disorder that is characterized by craving for tobacco products, withdrawal upon smoking cessation, and relapse after periods of abstinence. Previous studies demonstrated that systemic administration of α2-adrenergic receptor agonists attenuates stress-induced reinstatement of drug seeking in rats. The aim of the present experiments was to investigate the role of noradrenergic transmission in the central nucleus of amygdala (CeA) in stress-induced reinstatement of nicotine seeking. Rats self-administered nicotine for 14-16 days and then nicotine seeking was extinguished by substituting saline for nicotine. The effect of the intra-CeA infusion of the α2-adrenergic receptor agonists clonidine and dexmedetomidine, the nonselective β1/β2-adrenergic receptor antagonist propranolol, and the α1-adrenergic receptor antagonist prazosin on stress-induced reinstatement of nicotine seeking was investigated. In all the experiments, exposure to footshocks reinstated extinguished nicotine seeking. The administration of clonidine or dexmedetomidine into the CeA attenuated stress-induced reinstatement of nicotine seeking. The administration of propranolol or prazosin into the CeA did not affect stress-induced reinstatement of nicotine seeking. Furthermore, intra-CeA administration of clonidine or dexmedetomidine did not affect operant responding for food pellets. This suggests that the effects of clonidine and dexmedetomidine on stress-induced reinstatement of nicotine seeking were not mediated by motor impairments or sedation. Taken together, these findings indicate that stimulation of α2-adrenergic receptors, but not blockade of α1 or β-adrenergic receptors, in the CeA attenuates stress-induced reinstatement of nicotine seeking. These findings suggest that α2-adrenergic receptor agonists may at least partly attenuate stress-induced reinstatement of nicotine seeking by stimulating α2-adrenergic receptors in the CeA.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures







Similar articles
-
Effects of the CRF receptor antagonist D-Phe CRF(12-41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats.Neuropharmacology. 2007 Dec;53(8):958-66. doi: 10.1016/j.neuropharm.2007.09.007. Epub 2007 Sep 23. Neuropharmacology. 2007. PMID: 17976662 Free PMC article.
-
Blockade of CRF1 receptors in the central nucleus of the amygdala attenuates the dysphoria associated with nicotine withdrawal in rats.Pharmacol Biochem Behav. 2012 Mar;101(1):62-8. doi: 10.1016/j.pbb.2011.12.001. Epub 2011 Dec 9. Pharmacol Biochem Behav. 2012. PMID: 22182462 Free PMC article.
-
A critical role for the melanocortin 4 receptor in stress-induced relapse to nicotine seeking in rats.Addict Biol. 2015 Mar;20(2):324-35. doi: 10.1111/adb.12129. Epub 2014 Feb 24. Addict Biol. 2015. PMID: 24612112
-
Neuropeptide systems and new treatments for nicotine addiction.Psychopharmacology (Berl). 2017 May;234(9-10):1419-1437. doi: 10.1007/s00213-016-4513-5. Epub 2016 Dec 28. Psychopharmacology (Berl). 2017. PMID: 28028605 Free PMC article. Review.
-
Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress.Neuropsychopharmacology. 2016 Jan;41(1):335-56. doi: 10.1038/npp.2015.142. Epub 2015 May 15. Neuropsychopharmacology. 2016. PMID: 25976297 Free PMC article. Review.
Cited by
-
Rewarding Effects of Nicotine Self-administration Increase Over Time in Male and Female Rats.Nicotine Tob Res. 2021 Nov 5;23(12):2117-2126. doi: 10.1093/ntr/ntab097. Nicotine Tob Res. 2021. PMID: 33987656 Free PMC article.
-
Anti-stress neuropharmacological mechanisms and targets for addiction treatment: A translational framework.Neurobiol Stress. 2018 Aug 11;9:84-104. doi: 10.1016/j.ynstr.2018.08.003. eCollection 2018 Nov. Neurobiol Stress. 2018. PMID: 30238023 Free PMC article. Review.
-
Pain, nicotine, and smoking: research findings and mechanistic considerations.Psychol Bull. 2011 Nov;137(6):1065-93. doi: 10.1037/a0025544. Psychol Bull. 2011. PMID: 21967450 Free PMC article. Review.
-
The role of guanfacine as a therapeutic agent to address stress-related pathophysiology in cocaine-dependent individuals.Adv Pharmacol. 2014;69:217-65. doi: 10.1016/B978-0-12-420118-7.00006-8. Adv Pharmacol. 2014. PMID: 24484979 Free PMC article. Review.
-
Effect of doxazosin on stress reactivity and the ability to resist smoking.J Psychopharmacol. 2017 Jul;31(7):830-840. doi: 10.1177/0269881117699603. Epub 2017 Apr 25. J Psychopharmacol. 2017. PMID: 28440105 Free PMC article.
References
-
- Ahmed SH, Koob GF. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology (Berl) 1997;132:289–295. - PubMed
-
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. - PubMed
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Press; Washington, DC: 2000.
-
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. - PubMed
-
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology (Berl) 1999;146:290–296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

