Age-related synapse loss in hippocampal CA3 is not reversed by caloric restriction
- PMID: 20854882
- PMCID: PMC2995889
- DOI: 10.1016/j.neuroscience.2010.09.022
Age-related synapse loss in hippocampal CA3 is not reversed by caloric restriction
Abstract
Caloric restriction (CR) is a reduction of total caloric intake without a decrease in micronutrients or a disproportionate reduction of any one dietary component. While CR attenuates age-related cognitive deficits in tasks of hippocampal-dependent memory, the cellular mechanisms by which CR improves this cognitive decline are poorly understood. Previously, we have reported age-related decreases in key synaptic proteins in the CA3 region of the hippocampus that are stabilized by lifelong CR. In the present study, we examined possible age-related changes in the functional microcircuitry of the synapses in the stratum lacunosum-molecular (SL-M) of the CA3 region of the hippocampus, and whether lifelong CR might prevent these age-related alterations. We used serial electron microscopy to reconstruct and classify SL-M synapses and their postsynaptic spines. We analyzed synapse number and size as well as spine surface area and volume in young (10 months) and old (29 months) ad libitum fed rats and in old rats that were calorically restricted from 4 months of age. We limited our analysis to SL-M because previous work demonstrated age-related decreases in synaptophysin confined to this specific layer and region of the hippocampus. The results revealed an age-related decrease in macular axo-spinous synapses that was not reversed by CR that occurred in the absence of changes in the size of synapses or spines. Thus, the benefits of CR for CA3 function and synaptic plasticity may involve other biological effects including the stabilization of synaptic proteins levels in the face of age-related synapse loss.
Copyright © 2010 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures


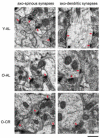
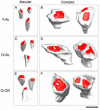
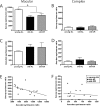
References
-
- Cabalka LM, Hyman BT, Goodlett CR, Ritchie TC, Van Hoesen GW. Alteration in the pattern of nerve terminal protein immunoreactivity in the perforant pathway in Alzheimer's disease and in rats after entorhinal lesions. Neurobiol Aging. 1992;13:283–291. - PubMed
-
- De Groot DMG, Bierman EPB. Numerical changes in rat hippocampal synapses. An effect of “aging”? Acta Stereologica. 1987;6:53–58.
-
- De RM, Klauser P, Garcia PM, Poglia L, Muller D. Spine dynamics and synapse remodeling during LTP and memory processes. Prog Brain Res. 2008;169:199–207. - PubMed
-
- Desmond NL, Levy WB. Changes in the postsynaptic density with long-term potentiation in the dentate gyrus. J Comp Neurol. 1986;253:476–482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

