The cell biology of vision
- PMID: 20855501
- PMCID: PMC3101587
- DOI: 10.1083/jcb.201006020
The cell biology of vision
Abstract
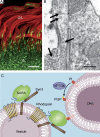
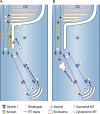
Humans possess the remarkable ability to perceive color, shape, and motion, and to differentiate between light intensities varied by over nine orders of magnitude. Phototransduction--the process in which absorbed photons are converted into electrical responses--is the first stage of visual processing, and occurs in the outer segment, the light-sensing organelle of the photoreceptor cell. Studies of genes linked to human inherited blindness have been crucial to understanding the biogenesis of the outer segment and membrane-trafficking of photoreceptors.
Figures
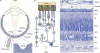


References
-
- Abd-El-Barr M.M., Sykoudis K., Andrabi S., Eichers E.R., Pennesi M.E., Tan P.L., Wilson J.H., Katsanis N., Lupski J.R., Wu S.M. 2007. Impaired photoreceptor protein transport and synaptic transmission in a mouse model of Bardet-Biedl syndrome. Vision Res. 47:3394–3407 10.1016/j.visres.2007.09.016 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

