Defining the mechanism of polymerization in the serpinopathies
- PMID: 20855577
- PMCID: PMC2951428
- DOI: 10.1073/pnas.1004785107
Defining the mechanism of polymerization in the serpinopathies
Abstract
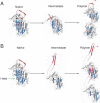
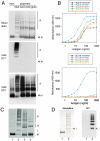
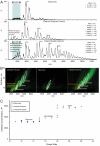
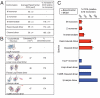
The serpinopathies result from the ordered polymerization of mutants of members of the serine proteinase inhibitor (serpin) superfamily. These polymers are retained within the cell of synthesis where they cause a toxic gain of function. The serpinopathies are exemplified by inclusions that form with the common severe Z mutant of α(1)-antitrypsin that are associated with liver cirrhosis. There is considerable controversy as to the pathway of serpin polymerization and the structure of pathogenic polymers that cause disease. We have used synthetic peptides, limited proteolysis, monoclonal antibodies, and ion mobility-mass spectrometry to characterize the polymerogenic intermediate and pathological polymers formed by Z α(1)-antitrypsin. Our data are best explained by a model in which polymers form through a single intermediate and with a reactive center loop-β-sheet A linkage. Our data are not compatible with the recent model in which polymers are linked by a β-hairpin of the reactive center loop and strand 5A. Understanding the structure of the serpin polymer is essential for rational drug design strategies that aim to block polymerization and so treat α(1)-antitrypsin deficiency and the serpinopathies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gooptu B, Lomas DA. Conformational pathology of the serpins: themes, variations and therapeutic strategies. Annu Rev Biochem. 2009;78:147–176. - PubMed
-
- Sveger T. The natural history of liver disease in α1-antitrypsin deficient children. Acta Paediatr Scand. 1988;77:847–851. - PubMed
-
- Lomas DA, Evans DL, Finch JT, Carrell RW. The mechanism of Z α1-antitrypsin accumulation in the liver. Nature. 1992;357:605–607. - PubMed
-
- James EL, Bottomley SP. The mechanism of α1-antitrypsin polymerization probed by fluorescence spectroscopy. Arch Biochem Biophys. 1998;356:296–300. - PubMed
-
- Dafforn TR, Mahadeva R, Elliott PR, Sivasothy P, Lomas DA. A kinetic mechanism for the polymerization of α1-antitrypsin. J Biol Chem. 1999;274:9548–9555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

