Complement receptor 1 is the host erythrocyte receptor for Plasmodium falciparum PfRh4 invasion ligand
- PMID: 20855594
- PMCID: PMC2951459
- DOI: 10.1073/pnas.1008151107
Complement receptor 1 is the host erythrocyte receptor for Plasmodium falciparum PfRh4 invasion ligand
Abstract
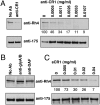
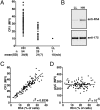
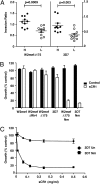
Plasmodium falciparum is responsible for the most severe form of malaria disease in humans, causing more than 1 million deaths each year. As an obligate intracellular parasite, P. falciparum's ability to invade erythrocytes is essential for its survival within the human host. P. falciparum invades erythrocytes using multiple host receptor-parasite ligand interactions known as invasion pathways. Here we show that CR1 is the host erythrocyte receptor for PfRh4, a major P. falciparum ligand essential for sialic acid-independent invasion. PfRh4 and CR1 interact directly, with a K(d) of 2.9 μM. PfRh4 binding is strongly correlated with the CR1 level on the erythrocyte surface. Parasite invasion via sialic acid-independent pathways is reduced in low-CR1 erythrocytes due to limited availability of this receptor on the surface. Furthermore, soluble CR1 can competitively block binding of PfRh4 to the erythrocyte surface and specifically inhibit sialic acid-independent parasite invasion. These results demonstrate that CR1 is an erythrocyte receptor used by the parasite ligand PfRh4 for P. falciparum invasion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–766. - PubMed
-
- Peterson DS, Wellems TE. EBL-1, a putative erythrocyte-binding protein of Plasmodium falciparum, maps within a favored linkage group in two genetic crosses. Mol Biochem Parasitol. 2000;105:105–113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

