Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis
- PMID: 20855599
- PMCID: PMC2951450
- DOI: 10.1073/pnas.1010811107
Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis
Abstract
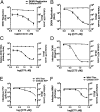
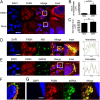
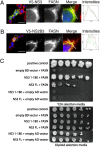
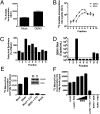
Dengue virus (DENV) modifies cellular membranes to establish its sites of replication. Although the 3D architecture of these structures has recently been described, little is known about the cellular pathways required for their formation and expansion. In this report, we examine the host requirements for DENV replication using a focused RNAi analysis combined with validation studies using pharmacological inhibitors. This approach identified three cellular pathways required for DENV replication: autophagy, actin polymerization, and fatty acid biosynthesis. Further characterization of the viral modulation of fatty acid biosynthesis revealed that a key enzyme in this pathway, fatty acid synthase (FASN), is relocalized to sites of DENV replication. DENV nonstructural protein 3 (NS3) is responsible for FASN recruitment, inasmuch as (i) NS3 expressed in the absence of other viral proteins colocalizes with FASN and (ii) NS3 interacts with FASN in a two-hybrid assay. There is an associated increase in the rate of fatty acid biosynthesis in DENV-infected cells, and de novo synthesized lipids preferentially cofractionate with DENV RNA. Finally, purified recombinant NS3 stimulates the activity of FASN in vitro. Taken together, these experiments suggest that DENV co-opts the fatty acid biosynthetic pathway to establish its replication complexes. This study provides mechanistic insight into DENV membrane remodeling and highlights the potential for the development of therapeutics that inhibit DENV replication by targeting the fatty acid biosynthetic pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Greasing the wheels of replication.Nat Rev Microbiol. 2010 Nov;8(11):758. doi: 10.1038/nrmicro2463. Nat Rev Microbiol. 2010. PMID: 21080554 No abstract available.
References
-
- Halstead SB. Dengue. Lancet. 2007;370:1644–1652. - PubMed
-
- Miller S, Kastner S, Krijnse-Locker J, Bühler S, Bartenschlager R. The non-structural protein 4A of dengue virus is an integral membrane protein inducing membrane alterations in a 2K-regulated manner. J Biol Chem. 2007;282:8873–8882. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

