Mutations of cellulose synthase (CESA1) phosphorylation sites modulate anisotropic cell expansion and bidirectional mobility of cellulose synthase
- PMID: 20855602
- PMCID: PMC2951445
- DOI: 10.1073/pnas.1012348107
Mutations of cellulose synthase (CESA1) phosphorylation sites modulate anisotropic cell expansion and bidirectional mobility of cellulose synthase
Abstract
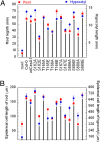
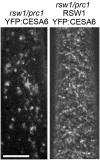
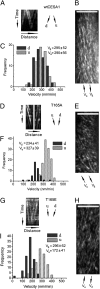
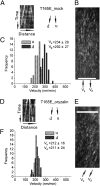
The CESA1 component of cellulose synthase is phosphorylated at sites clustered in two hypervariable regions of the protein. Mutations of the phosphorylated residues to Ala (A) or Glu (E) alter anisotropic cell expansion and cellulose synthesis in rapidly expanding roots and hypocotyls. Expression of T166E, S686E, or S688E mutants of CESA1 fully rescued the temperature sensitive cesA1-1 allele (rsw1) at a restrictive temperature whereas mutations to A at these positions caused defects in anisotropic cell expansion. However, mutations to E at residues surrounding T166 (i.e., S162, T165, and S167) caused opposite effects. Live-cell imaging of fluorescently labeled CESA showed close correlations between tissue or cell morphology and patterns of bidirectional motility of CESA complexes in the plasma membrane. In the WT, CESA complexes moved at similar velocities in both directions along microtubule tracks. By contrast, the rate of movement of CESA particles was directionally asymmetric in mutant lines that exhibited abnormal tissue or cell expansion, and the asymmetry was removed upon depolymerizing microtubules with oryzalin. This suggests that phosphorylation of CESA differentially affects a polar interaction with microtubules that may regulate the length or quantity of a subset of cellulose microfibrils and that this, in turn, alters microfibril structure in the primary cell wall resulting in or contributing to the observed defect in anisotropic cell expansion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
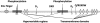
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

