Lateral opening of a translocon upon entry of protein suggests the mechanism of insertion into membranes
- PMID: 20855604
- PMCID: PMC2951439
- DOI: 10.1073/pnas.1012556107
Lateral opening of a translocon upon entry of protein suggests the mechanism of insertion into membranes
Abstract
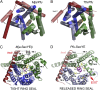
The structure of the protein-translocating channel SecYEβ from Pyrococcus furiosus at 3.1-Å resolution suggests a mechanism for chaperoning transmembrane regions of a protein substrate during its lateral delivery into the lipid bilayer. Cytoplasmic segments of SecY orient the C-terminal α-helical region of another molecule, suggesting a general binding mode and a promiscuous guiding surface capable of accommodating diverse nascent chains at the exit of the ribosomal tunnel. To accommodate this putative nascent chain mimic, the cytoplasmic vestibule widens, and a lateral exit portal is opened throughout its entire length for partition of transmembrane helical segments to the lipid bilayer. In this primed channel, the central plug still occludes the pore while the lateral gate is opened, enabling topological arbitration during early protein insertion. In vivo, a 15 amino acid truncation of the cytoplasmic C-terminal helix of SecY fails to rescue a secY-deficient strain, supporting the essential role of this helix as suggested from the structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- White SH, von Heijne G. The machinery of membrane protein assembly. Curr Opin Struct Biol. 2004;14:397–404. - PubMed
-
- Brundage L, Hendrick JP, Schiebel E, Driessen AJ, Wickner W. The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell. 1990;62:649–657. - PubMed
-
- Meyer TH, et al. The bacterial SecY/E translocation complex forms channel-like structures similar to those of the eukaryotic Sec61p complex. J Mol Biol. 1999;285:1789–1800. - PubMed
-
- Egea PF, Stroud RM, Walter P. Targeting proteins to membranes: Structure of the signal recognition particle. Curr Opin Struct Biol. 2005;15:213–220. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

