Soluble amyloid precursor protein (APP) regulates transthyretin and Klotho gene expression without rescuing the essential function of APP
- PMID: 20855613
- PMCID: PMC2951422
- DOI: 10.1073/pnas.1012568107
Soluble amyloid precursor protein (APP) regulates transthyretin and Klotho gene expression without rescuing the essential function of APP
Erratum in
- Proc Natl Acad Sci U S A. 2013 Aug 6;110(32):13228
Abstract
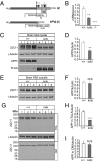
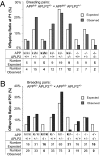
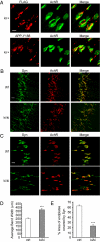
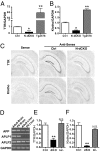
Amyloidogenic processing of the amyloid precursor protein (APP) generates a large secreted ectodomain fragment (APPsβ), β-amyloid (Aβ) peptides, and an APP intracellular domain (AICD). Whereas Aβ is viewed as critical for Alzheimer's disease pathogenesis, the role of other APP processing products remains enigmatic. Of interest, the AICD has been implicated in transcriptional regulation, and N-terminal cleavage of APPsβ has been suggested to produce an active fragment that may mediate axonal pruning and neuronal cell death. We previously reported that mice deficient in APP and APP-like protein 2 (APLP2) exhibit early postnatal lethality and neuromuscular synapse defects, whereas mice with neuronal conditional deletion of APP and APLP2 are viable. Using transcriptional profiling, we now identify transthyretin (TTR) and Klotho as APP/APLP2-dependent genes whose expression is decreased in loss-of-function states but increased in gain-of-function states. Significantly, by creating an APP knockin allele that expresses only APPsβ protein, we demonstrate that APPsβ is not normally cleaved in vivo and is fully capable of mediating the APP-dependent regulation of TTR and Klotho gene expression. Despite being an active regulator of gene expression, APPsβ did not rescue the lethality and neuromuscular synapse defects of APP and APLP2 double-KO animals. Our studies identify TTR and Klotho as physiological targets of APP that are regulated by soluble APPsβ independent of developmental APP functions. This unexpected APP-mediated signaling pathway may play an important role in maintaining TTR and Klotho levels and their respective functions in Aβ sequestration and aging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Turner PR, O'Connor K, Tate WP, Abraham WC. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol. 2003;70:1–32. - PubMed
-
- Cao X, Südhof TC. A transcriptionally active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–120. - PubMed
-
- Cao X, Südhof TC. Dissection of amyloid-beta precursor protein-dependent transcriptional transactivation. J Biol Chem. 2004;279:24601–24611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

