Oscillatory phase coupling coordinates anatomically dispersed functional cell assemblies
- PMID: 20855620
- PMCID: PMC2951408
- DOI: 10.1073/pnas.1008306107
Oscillatory phase coupling coordinates anatomically dispersed functional cell assemblies
Abstract
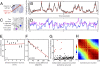
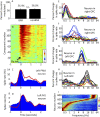
Hebb proposed that neuronal cell assemblies are critical for effective perception, cognition, and action. However, evidence for brain mechanisms that coordinate multiple coactive assemblies remains lacking. Neuronal oscillations have been suggested as one possible mechanism for cell assembly coordination. Prior studies have shown that spike timing depends upon local field potential (LFP) phase proximal to the cell body, but few studies have examined the dependence of spiking on distal LFP phases in other brain areas far from the neuron or the influence of LFP-LFP phase coupling between distal areas on spiking. We investigated these interactions by recording LFPs and single-unit activity using multiple microelectrode arrays in several brain areas and then used a unique probabilistic multivariate phase distribution to model the dependence of spike timing on the full pattern of proximal LFP phases, distal LFP phases, and LFP-LFP phase coupling between electrodes. Here we show that spiking activity in single neurons and neuronal ensembles depends on dynamic patterns of oscillatory phase coupling between multiple brain areas, in addition to the effects of proximal LFP phase. Neurons that prefer similar patterns of phase coupling exhibit similar changes in spike rates, whereas neurons with different preferences show divergent responses, providing a basic mechanism to bind different neurons together into coordinated cell assemblies. Surprisingly, phase-coupling-based rate correlations are independent of interneuron distance. Phase-coupling preferences correlate with behavior and neural function and remain stable over multiple days. These findings suggest that neuronal oscillations enable selective and dynamic control of distributed functional cell assemblies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kandel E. Principles of Neural Science. 4th Ed. New York: McGraw–Hill; 2000.
-
- Destexhe A, Rudolph M, Paré D. The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci. 2003;4:739–751. - PubMed
-
- Shepherd GM, Stepanyants A, Bureau I, Chklovskii D, Svoboda K. Geometric and functional organization of cortical circuits. Nat Neurosci. 2005;8:782–790. - PubMed
-
- Douglas RJ, Martin KAC. Neuronal circuits of the neocortex. Annu Rev Neurosci. 2004;27:419–451. - PubMed
-
- Hebb DO. The Organization of Behavior. New York: Wiley; 1949.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

