Molecular characterization of off-target activities of telithromycin: a potential role for nicotinic acetylcholine receptors
- PMID: 20855733
- PMCID: PMC2981250
- DOI: 10.1128/AAC.00840-10
Molecular characterization of off-target activities of telithromycin: a potential role for nicotinic acetylcholine receptors
Abstract
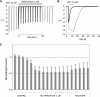
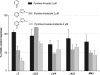
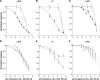
Adverse effects have limited the clinical use of telithromycin. Preferential inhibition of the nicotinic acetylcholine receptors (nAChR) at the neuromuscular junction (α3β2 and NMJ), the ciliary ganglion of the eye (α3β4 and α7), and the vagus nerve innervating the liver (α7) could account for the exacerbation of myasthenia gravis, the visual disturbance, and the liver failure seen with telithromycin use. The studies presented here enable the prediction of expected side effects of macrolides in development, such as solithromycin (CEM-101).
Figures
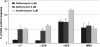
References
-
- Dani, J. A., and D. Bertrand. 2007. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 47:699-729. - PubMed
-
- Hogg, R. C., F. Bandelier, A. Benoit, R. Dosch, and D. Bertrand. 2008. An automated system for intracellular and intranuclear injection. J. Neurosci. Methods 169:65-75. - PubMed
-
- Jarvis, L. M. 2008. The Ketek effect. Chem. Eng. News 86:861-862.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

