Acute ablation of Langerhans cells enhances skin immune responses
- PMID: 20855870
- PMCID: PMC3050031
- DOI: 10.4049/jimmunol.1001802
Acute ablation of Langerhans cells enhances skin immune responses
Abstract
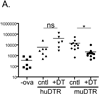
Understanding the function of Langerhans cells (LCs) in vivo has been complicated by conflicting results from LC-deficient mice. Human Langerin-DTA mice constitutively lack LCs and develop exaggerated contact hypersensitivity (CHS) responses. Murine Langerin-diphtheria toxin receptor (DTR) mice allow for the inducible elimination of LCs and Langerin(+) dermal dendritic cells (dDCs) after administration of diphtheria toxin, which results in reduced CHS. When Langerin(+) dDCs have partially repopulated the skin but LCs are still absent, CHS returns to normal. Thus, LCs appear to be suppressive in human Langerin-DTA mice and redundant in murine Langerin-DTR mice. To determine whether inducible versus constitutive LC ablation explains these results, we engineered human Langerin-DTR mice in which diphtheria toxin ablates LCs without affecting Langerin(+) dDCs. The inducible ablation of LCs in human Langerin-DTR mice resulted in increased CHS. Thus, LC-mediated suppression does not require their absence during ontogeny or during the steady-state and is consistent with a model in which LCs actively suppress Ag-specific CHS responses.
Figures



References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Romani N, Ebner S, Tripp CH, Flacher V, Koch F, Stoitzner P. Epidermal Langerhans cells--changing views on their function in vivo. Immunol Lett. 2006;106:119–125. - PubMed
-
- Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol. 2008;38:2369–2376. - PubMed
-
- Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B. Dynamics and Function of Langerhans Cells In Vivo Dermal Dendritic Cells Colonize Lymph Node AreasDistinct from Slower Migrating Langerhans Cells. Immunity. 2005;22:643–654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

