IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation
- PMID: 20855874
- PMCID: PMC3104023
- DOI: 10.4049/jimmunol.1002046
IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation
Abstract
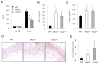
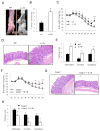
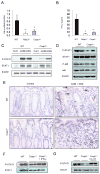
Colorectal cancer is a leading cause of cancer-related deaths worldwide. Chronic inflammation is recognized as a predisposing factor for the development of colon cancer, but the molecular mechanisms linking inflammation and tumorigenesis have remained elusive. Recent studies revealed a crucial role for the NOD-like receptor protein Nlrp3 in regulating inflammation through the assembly of proinflammatory protein complexes termed inflammasomes. However, its role in colorectal tumor formation remains unclear. In this study, we showed that mice deficient for Nlrp3 or the inflammasome effector caspase-1 were highly susceptible to azoxymethane/dextran sodium sulfate-induced inflammation and suffered from dramatically increased tumor burdens in the colon. This was a consequence of markedly reduced IL-18 levels in mice lacking components of the Nlrp3 inflammasome, which led to impaired production and activation of the tumor suppressors IFN-γ and STAT1, respectively. Thus, IL-18 production downstream of the Nlrp3 inflammasome is critically involved in protection against colorectal tumorigenesis.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures



References
-
- Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–1233. - PubMed
-
- Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

