CXCR3-B can mediate growth-inhibitory signals in human renal cancer cells by down-regulating the expression of heme oxygenase-1
- PMID: 20855888
- PMCID: PMC2978613
- DOI: 10.1074/jbc.M110.170324
CXCR3-B can mediate growth-inhibitory signals in human renal cancer cells by down-regulating the expression of heme oxygenase-1
Abstract
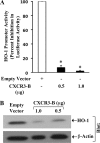
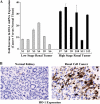
The chemokine receptor CXCR3 may play a critical role in the growth and metastasis of tumor cells, including renal tumors. It has been shown that CXCR3 has two splice variants with completely opposite functions; CXCR3-A promotes cell proliferation, whereas CXCR3-B inhibits cell growth. We recently demonstrated that the expression of growth-promoting CXCR3-A is up-regulated, and the growth-inhibitory CXCR3-B is markedly down-regulated in human renal cancer tissues; and the overexpression of CXCR3-B in renal cancer cells can significantly inhibit cell proliferation. However, the growth-inhibitory signal(s) through CXCR3-B are not well characterized. Here, we investigated the effector molecule(s) involved in CXCR3-B-mediated signaling events. We found that the overexpression of CXCR3-B in human renal cancer cells (Caki-1) promoted cellular apoptosis as observed by FACS analysis through Annexin-V staining. To examine whether the overexpression of CXCR3-B could alter the expression of any apoptosis-related genes in renal cancer cells, we performed a protein array. We found that CXCR3-B overexpression significantly down-regulated the expression of antiapoptotic heme oxygenase-1 (HO-1). By utilizing a HO-1 promoter-luciferase plasmid, we showed that CXCR3-B-mediated down-regulation of HO-1 was controlled at the transcriptional level as observed by luciferase assay. We also demonstrated that the inhibition of HO-1 expression using siRNA promoted apoptosis of renal cancer cells. Finally, we observed that human renal cancer tissues expressing low amounts of CXCR3-B significantly overexpress HO-1 at both mRNA and protein level. Together, we suggest that the overexpression of CXCR3-B may prevent the growth of renal tumors through the inhibition of antiapoptotic HO-1.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

