Human AP endonuclease (APE1/Ref-1) and its acetylation regulate YB-1-p300 recruitment and RNA polymerase II loading in the drug-induced activation of multidrug resistance gene MDR1
- PMID: 20856196
- PMCID: PMC3010319
- DOI: 10.1038/onc.2010.435
Human AP endonuclease (APE1/Ref-1) and its acetylation regulate YB-1-p300 recruitment and RNA polymerase II loading in the drug-induced activation of multidrug resistance gene MDR1
Abstract
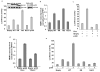
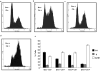
The overexpression of human apurinic/apyrimidinic (AP) endonuclease 1 (APE1/Ref-1), a key enzyme in the DNA base excision repair (BER) pathway, is often associated with tumor cell resistance to various anticancer drugs. In this study, we examined the molecular basis of transcriptional regulatory (nonrepair) function of APE1 in promoting resistance to certain types of drugs. We have recently shown that APE1 stably interacts with Y-box-binding protein 1 (YB-1), and acts as its coactivator for the expression of multidrug resistance gene MDR1, thereby causing drug resistance. In this study, we show, to the best of our knowledge, for the first time that APE1 is stably associated with the basic transcription factor RNA polymerase II (RNA pol II) and the coactivator p300 on the endogenous MDR1 promoter. The depletion of APE1 significantly reduces YB-1-p300 recruitment to the promoter, resulting in reduced RNA pol II loading. Drug-induced APE1 acetylation, which is mediated by p300, enhances formation of acetylated APE1 (AcAPE1)-YB-1-p300 complex on the MDR1 promoter. Enhanced recruitment of this complex increases MDR1 promoter-dependent luciferase activity and its endogenous expression. Using APE1-downregulated cells and cells overexpressing wild-type APE1 or its nonacetylable mutant, we have demonstrated that the loss of APE1's acetylation impaired MDR1 activation and sensitizes the cells to cisplatin or etoposide. We have thus established the basis for APE1's acetylation-dependent regulatory function in inducing MDR1-mediated drug resistance.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures

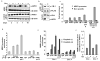


References
-
- Ahmad S, Glazer RI. Expression of the antisense cDNA for protein kinase C alpha attenuates resistance in doxorubicin-resistant MCF-7 breast carcinoma cells. Mol Pharmacol. 1993;43:858–862. - PubMed
-
- Ahmad S, Safa AR, Glazer RI. Modulation of P-glycoprotein by protein kinase C alpha in a baculovirus expression system. Biochemistry. 1994;33:10313–10318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

