Azelastine hydrochloride, a dual-acting anti-inflammatory ophthalmic solution, for treatment of allergic conjunctivitis
- PMID: 20856595
- PMCID: PMC2938280
- DOI: 10.2147/opth.s13479
Azelastine hydrochloride, a dual-acting anti-inflammatory ophthalmic solution, for treatment of allergic conjunctivitis
Abstract
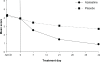
Over 50% of patients who seek treatment for allergies present with ocular symptoms. Our current ability to control ocular allergic symptoms is greater than ever before. Newer dual-acting topical eyedrops attack multiple facets of the allergic cascade. Azelastine has antihistaminic effects providing immediate relief, mast cell stabilization providing early-phase intervention, and inhibition of expression and activation of anti-inflammatory mediators which characterize the late phase of the immune reaction. The ophthalmic eyedrop formulation is approved for treatment of allergic conjunctivitis in adults and children aged over 3 years. In clinical trials comparing azelastine with other dual-acting eyedrops, such as levocabastine and olopatadine, azelastine was reported to be slightly less efficacious and to sting briefly upon administration. Even so, many patients experienced the full benefit of symptom relief, and preferred azelastine. As a broad-spectrum drug, azelastine offers many desirable properties for management of ocular allergies. Because it can often produce maximal effect with just twice-daily dosing, azelastine is a particularly good choice for the allergic population in whom minimizing exposure to topical products and preservatives is a key concern.
Keywords: H1 receptor antagonism; allergic conjunctivitis; dual acting anti-inflammatory; inflammatory mediator inhibition; mast cell stabilization.
Figures

Similar articles
-
Clinical and Biological Evaluation of NAAGA Versus Azelastine Eye Drops in Patients with Allergic Conjunctivitis and Tear Film Dysfunction: A Randomized Controlled Trial.Ophthalmol Ther. 2025 Jul;14(7):1581-1595. doi: 10.1007/s40123-025-01171-6. Epub 2025 Jun 2. Ophthalmol Ther. 2025. PMID: 40455383 Free PMC article.
-
Efficacy and tolerability of newer antihistamines in the treatment of allergic conjunctivitis.Drugs. 2005;65(2):215-28. doi: 10.2165/00003495-200565020-00004. Drugs. 2005. PMID: 15631542 Review.
-
Evaluation of the efficacy of olopatadine hydrochloride 0.1% ophthalmic solution and azelastine hydrochloride 0.05% ophthalmic solution in the conjunctival allergen challenge model.Clin Ther. 2001 Aug;23(8):1272-80. doi: 10.1016/s0149-2918(01)80106-5. Clin Ther. 2001. PMID: 11558863 Clinical Trial.
-
Randomized, double-masked comparison of olopatadine ophthalmic solution, mometasone furoate monohydrate nasal spray, and fexofenadine hydrochloride tablets using the conjunctival and nasal allergen challenge models.Clin Ther. 2003 Aug;25(8):2245-67. doi: 10.1016/s0149-2918(03)80217-5. Clin Ther. 2003. PMID: 14512132 Clinical Trial.
-
Intranasal azelastine. A review of its efficacy in the management of allergic rhinitis.Drugs. 1998 Jul;56(1):91-114. doi: 10.2165/00003495-199856010-00011. Drugs. 1998. PMID: 9664202 Review.
Cited by
-
Managing Allergic Rhinitis in the Pharmacy: An ARIA Guide for Implementation in Practice.Pharmacy (Basel). 2020 May 16;8(2):85. doi: 10.3390/pharmacy8020085. Pharmacy (Basel). 2020. PMID: 32429362 Free PMC article.
-
Ocular redness - II: Progress in development of therapeutics for the management of conjunctival hyperemia.Ocul Surf. 2021 Jul;21:66-77. doi: 10.1016/j.jtos.2021.05.004. Epub 2021 May 15. Ocul Surf. 2021. PMID: 34000363 Free PMC article. Review.
-
Second-Generation Histamine H1 Receptor Antagonists Suppress Delayed Rectifier K+-Channel Currents in Murine Thymocytes.Biomed Res Int. 2019 Apr 30;2019:6261951. doi: 10.1155/2019/6261951. eCollection 2019. Biomed Res Int. 2019. Retraction in: Biomed Res Int. 2024 Jan 9;2024:9842701. doi: 10.1155/2024/9842701. PMID: 31183371 Free PMC article. Retracted.
-
Clinical and Biological Evaluation of NAAGA Versus Azelastine Eye Drops in Patients with Allergic Conjunctivitis and Tear Film Dysfunction: A Randomized Controlled Trial.Ophthalmol Ther. 2025 Jul;14(7):1581-1595. doi: 10.1007/s40123-025-01171-6. Epub 2025 Jun 2. Ophthalmol Ther. 2025. PMID: 40455383 Free PMC article.
-
A double-blind, placebo-controlled trial of the efficacy and safety of two doses of azelastine hydrochloride in perennial allergic rhinitis.Front Allergy. 2023 Oct 18;4:1244012. doi: 10.3389/falgy.2023.1244012. eCollection 2023. Front Allergy. 2023. PMID: 37920410 Free PMC article.
References
-
- Bielory L, Bielory B. Ocular allergy: An allergist’s perspective. Available at: http://bmctoday.net/advancedocularcare/2010/03/article.asp?f=ocular-alle.... Accessed Aug 16, 2010.
-
- Pflugfelder SC. Prevalence, burden, and pharmacoeconomics of dry eye disease. Am J Manag Care. 2008;14(Suppl 3):S102–S106. - PubMed
-
- Bielory L, Lien KW, Bigelsen S. Efficacy and tolerability of newer antihistamines in the treatment of allergic conjunctivitis. Drugs. 2005;65:215–218. - PubMed
-
- Bielory L, Buddiga P, Bigelsen S. Ocular allergy treatment comparisons: Azelastine and olopatadine. Curr Allergy Asthma Rep. 2004;4:320–325. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

