Mammalian Otolin: a multimeric glycoprotein specific to the inner ear that interacts with otoconial matrix protein Otoconin-90 and Cerebellin-1
- PMID: 20856818
- PMCID: PMC2939893
- DOI: 10.1371/journal.pone.0012765
Mammalian Otolin: a multimeric glycoprotein specific to the inner ear that interacts with otoconial matrix protein Otoconin-90 and Cerebellin-1
Abstract
Background: The mammalian otoconial membrane is a dense extracellular matrix containing bio-mineralized otoconia. This structure provides the mechanical stimulus necessary for hair cells of the vestibular maculae to respond to linear accelerations and gravity. In teleosts, Otolin is required for the proper anchoring of otolith crystals to the sensory maculae. Otoconia detachment and subsequent entrapment in the semicircular canals can result in benign paroxysmal positional vertigo (BPPV), a common form of vertigo for which the molecular basis is unknown. Several cDNAs encoding protein components of the mammalian otoconia and otoconial membrane have recently been identified, and mutations in these genes result in abnormal otoconia formation and balance deficits.
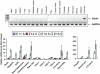
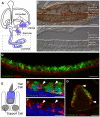
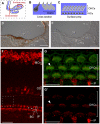
Principal findings: Here we describe the cloning and characterization of mammalian Otolin, a protein constituent of otoconia and the otoconial membrane. Otolin is a secreted glycoprotein of ∼70 kDa, with a C-terminal globular domain that is homologous to the immune complement C1q, and contains extensive posttranslational modifications including hydroxylated prolines and glycosylated lysines. Like all C1q/TNF family members, Otolin multimerizes into higher order oligomeric complexes. The expression of otolin mRNA is restricted to the inner ear, and immunohistochemical analysis identified Otolin protein in support cells of the vestibular maculae and semi-circular canal cristae. Additionally, Otolin forms protein complexes with Cerebellin-1 and Otoconin-90, two protein constituents of the otoconia, when expressed in vitro. Otolin was also found in subsets of support cells and non-sensory cells of the cochlea, suggesting that Otolin is also a component of the tectorial membrane.
Conclusion: Given the importance of Otolin in lower organisms, the molecular cloning and biochemical characterization of the mammalian Otolin protein may lead to a better understanding of otoconial development and vestibular dysfunction.
Conflict of interest statement
Figures






References
-
- Torres M, Giraldez F. The development of the vertebrate inner ear. Mech Dev. 1998;71:5–21. - PubMed
-
- Whitfield TT, Riley BB, Chiang MY, Phillips B. Development of the zebrafish inner ear. Dev Dyn. 2002;223:427–458. - PubMed
-
- Legan PK, Rau A, Keen JN, Richardson GP. The mouse tectorins. Modular matrix proteins of the inner ear homologous to components of the sperm-egg adhesion system. J Biol Chem. 1997;272:8791–8801. - PubMed
-
- Rau A, Legan PK, Richardson GP. Tectorin mRNA expression is spatially and temporally restricted during mouse inner ear development. J Comp Neurol. 1999;405:271–280. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

