Ghrelin is produced in taste cells and ghrelin receptor null mice show reduced taste responsivity to salty (NaCl) and sour (citric acid) tastants
- PMID: 20856820
- PMCID: PMC2939079
- DOI: 10.1371/journal.pone.0012729
Ghrelin is produced in taste cells and ghrelin receptor null mice show reduced taste responsivity to salty (NaCl) and sour (citric acid) tastants
Abstract
Background: The gustatory system plays a critical role in determining food preferences, food intake and energy balance. The exact mechanisms that fine tune taste sensitivity are currently poorly defined, but it is clear that numerous factors such as efferent input and specific signal transduction cascades are involved.
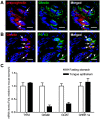
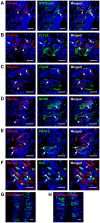
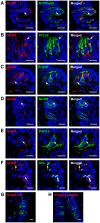
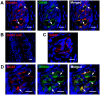
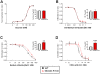
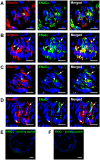
Methodology/principal findings: Using immunohistochemical analyses, we show that ghrelin, a hormone classically considered to be an appetite-regulating hormone, is present within the taste buds of the tongue. Prepro-ghrelin, prohormone convertase 1/3 (PC 1/3), ghrelin, its cognate receptor (GHSR), and ghrelin-O-acyltransferase (GOAT , the enzyme that activates ghrelin) are expressed in Type I, II, III and IV taste cells of mouse taste buds. In addition, ghrelin and GHSR co-localize in the same taste cells, suggesting that ghrelin works in an autocrine manner in taste cells. To determine a role for ghrelin in modifying taste perception, we performed taste behavioral tests using GHSR null mice. GHSR null mice exhibited significantly reduced taste responsivity to sour (citric acid) and salty (sodium chloride) tastants.
Conclusions/significance: These findings suggest that ghrelin plays a local modulatory role in determining taste bud signaling and function and could be a novel mechanism for the modulation of salty and sour taste responsivity.
Conflict of interest statement
Figures
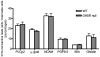
References
-
- Scott K. Taste recognition: food for thought. Neuron. 2005;48:455–464. - PubMed
-
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. - PubMed
-
- Pumplin DW, Yu C, Smith DV. Light and dark cells of rat vallate taste buds are morphologically distinct cell types. J Comp Neurol. 1997;378:389–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

