Tiotropium bromide inhibits TGF-β-induced MMP production from lung fibroblasts by interfering with Smad and MAPK pathways in vitro
- PMID: 20856827
- PMCID: PMC2939683
- DOI: 10.2147/copd.s11737
Tiotropium bromide inhibits TGF-β-induced MMP production from lung fibroblasts by interfering with Smad and MAPK pathways in vitro
Abstract
Background: chronic obstructive pulmonary disease (COPD) is characterized by chronic inflammation and structural alterations (ie, tissue remodeling) throughout the conducting airways, parenchyma, and pulmonary vasculature. Matrix metalloproteinases (MMPs) are extracellular degrading enzymes that play a critical role in inflammatory cell infiltration and tissue remodeling, but the influence of the agents that are used for the treatment of COPD on the production of MMPs is not well understood.
Purpose: the present study aimed to examine the influence of tiotropium bromide hydrate (TBH) on the production of MMPs from lung fibroblasts (LFs) induced by transforming growth factor (TGF)-β in vitro.
Methods: LFs, at a concentration of 5 × 10(5) cells·mL(-1), were stimulated with TGF-β in the presence of various concentrations of TBH. MMP-1 and MMP-2 levels in culture supernatants were examined by enzyme-linked immunosorbent assay (ELISA), and MMP messenger ribonucleic acid (mRNA) expression was examined by real-time polymerase chain reaction (RT-PCR). The influence of TBH on TGF-β signaling pathways was also analyzed by examining Smad activation and signaling protein phosphorylation by ELISA.
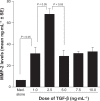
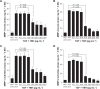
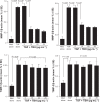
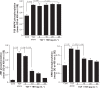
Results: TBH at more than 15 pg·mL(-1) inhibited the production of MMP-1 and MMP-2, but not tissue inhibitor of matrix metalloproteinase (TIMP)-1 and TIMP-2, from LFs, after TGF-β stimulation. TBH also suppressed MMP mRNA expression through the inhibition of Smad activation and signaling protein, extracellular-signal-regulated kinase (ERK) 1 and 2, and c-Jun N-terminal kinase (JNK), phosphorylation.
Conclusion: These results may suggest that TBH suppresses MMP production from LFs, through interference of TGF-β-mediated signaling pathways and results in favorable modification of the clinical status of COPD.
Keywords: TGF-β; in vitro; inhibition; lung fibroblast; matrix metalloproteinases; tiotropium bromide.
Figures
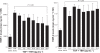
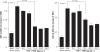
References
-
- Lowrey GE, Henderson N, Blakey JD, Corne JM, Johnson SR. MMP-9 protein level does not reflect overall MMP activity in the airways of patients with COPD. Respir Med. 2008;102:845–851. - PubMed
-
- Hamid Q, Cosio M, Lim S. Inflammation and remodeling in chronic obstructive disease. J Allergy Clin Immunol. 2004;114:1479–1481. - PubMed
-
- Jeffery PK. Remodeling and inflammation of bronchus in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:176–183. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

