Onset of action of indacaterol in patients with COPD: comparison with salbutamol and salmeterol-fluticasone
- PMID: 20856830
- PMCID: PMC2939686
- DOI: 10.2147/copd.s12120
Onset of action of indacaterol in patients with COPD: comparison with salbutamol and salmeterol-fluticasone
Abstract
Background: indacaterol is a novel, inhaled once-daily ultra-long-acting β(2)-agonist for the treatment of chronic obstructive pulmonary disease (COPD).
Objectives: this study compared the onset of action of single doses of indacaterol 150 and 300 μg with salbutamol 200 μg, salmeterol-fluticasone 50/500 μg, and placebo in moderate-to-severe COPD patients.
Methods: this was a multicenter, randomized, double-blind, placebo-controlled crossover study. The primary variable was forced expiratory volume in one second (FEV(1)) at five minutes postdose.
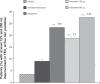
Results: out of 89 patients randomized (mean age 62 years), 86 completed the study. At five minutes postdose, both indacaterol doses were statistically and clinically superior to placebo (P < 0.001), with treatment-placebo differences in FEV(1) of 100 (95% confidence interval [CI] 70-130) mL and 120 (95% CI 90-150) mL for indacaterol 150 and 300 μg, respectively. FEV(1) at five minutes postdose with both indacaterol doses was numerically higher than for salbutamol (10 and 30 mL for indacaterol 150 and 300 μg, respectively) and significantly higher than for salmeterol-fluticasone (50 mL, P = 0.003; 70 mL, P < 0.001, respectively). Moreover, both indacaterol doses showed significantly higher FEV(1) than placebo (P < 0.001) at all postdose time points. The numbers of patients with an FEV(1) increase of at least 12% and 200 mL at five minutes postdose were 16 (18.8%), 24 (27.6%), 20 (23.3%), 8 (9.1%), and 3 (3.4%) for indacaterol 150 and 300 μg, salbutamol 200 μg, salmeterol-fluticasone 50/500 μg, and placebo, respectively.
Conclusions: single doses of indacaterol 150 and 300 μg demonstrated a fast onset of action similar to that for salbutamol and faster than that for salmeterol-fluticasone.
Keywords: chronic obstructive pulmonary disease; indacaterol; onset of action.
Figures
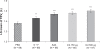



References
-
- Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: Current burden and future projections. Eur Respir J. 2006;27:397–412. - PubMed
-
- Global strategy for diagnosis, management, and prevention of COPD. Available at: http://www.goldcopd.com/Guidelineitem.asp?l1=2&l2=1&intId=2003. Accessed Mar 2, 2010.
-
- DiMatteo MR. Variations in patients’ adherence to medical recommendations: A quantitative review of 50 years of research. Med Care. 2004;42:200–209. - PubMed
-
- Rand CS. Non-adherence with asthma therapy: More than just forgetting. J Pediatr. 2005;146:157–159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

