A novel histone deacetylase inhibitor exhibits antitumor activity via apoptosis induction, F-actin disruption and gene acetylation in lung cancer
- PMID: 20856855
- PMCID: PMC2939045
- DOI: 10.1371/journal.pone.0012417
A novel histone deacetylase inhibitor exhibits antitumor activity via apoptosis induction, F-actin disruption and gene acetylation in lung cancer
Abstract
Background: Lung cancer is the leading cause of cancer mortality worldwide, yet the therapeutic strategy for advanced non-small cell lung cancer (NSCLC) is limitedly effective. In addition, validated histone deacetylase (HDAC) inhibitors for the treatment of solid tumors remain to be developed. Here, we propose a novel HDAC inhibitor, OSU-HDAC-44, as a chemotherapeutic drug for NSCLC.
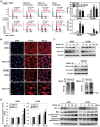
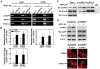
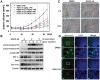
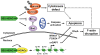
Methodology/principal findings: The cytotoxicity effect of OSU-HDAC-44 was examined in three human NSCLC cell lines including A549 (p53 wild-type), H1299 (p53 null), and CL1-1 (p53 mutant). The antiproliferative mechanisms of OSU-HDAC-44 were investigated by flow cytometric cell cycle analysis, apoptosis assays and genome-wide chromatin-immunoprecipitation-on-chip (ChIP-on-chip) analysis. Mice with established A549 tumor xenograft were treated with OSU-HDAC-44 or vehicle control and were used to evaluate effects on tumor growth, cytokinesis inhibition and apoptosis. OSU-HDAC-44 was a pan-HDAC inhibitor and exhibits 3-4 times more effectiveness than suberoylanilide hydroxamic acid (SAHA) in suppressing cell viability in various NSCLC cell lines. Upon OSU-HDAC-44 treatment, cytokinesis was inhibited and subsequently led to mitochondria-mediated apoptosis. The cytokinesis inhibition resulted from OSU-HDAC-44-mediated degradation of mitosis and cytokinesis regulators Auroroa B and survivin. The deregulation of F-actin dynamics induced by OSU-HDAC-44 was associated with reduction in RhoA activity resulting from srGAP1 induction. ChIP-on-chip analysis revealed that OSU-HDAC-44 induced chromatin loosening and facilitated transcription of genes involved in crucial signaling pathways such as apoptosis, axon guidance and protein ubiquitination. Finally, OSU-HDAC-44 efficiently inhibited A549 xenograft tumor growth and induced acetylation of histone and non-histone proteins and apoptosis in vivo.
Conclusions/significance: OSU-HDAC-44 significantly suppresses tumor growth via induction of cytokinesis defect and intrinsic apoptosis in preclinical models of NSCLC. Our data provide compelling evidence that OSU-HDAC-44 is a potent HDAC targeted inhibitor and can be tested for NSCLC chemotherapy.
Conflict of interest statement
Figures



References
-
- Danesi R, de Braud F, Fogli S, de Pas TM, Di Paolo A, et al. Pharmacogenetics of anticancer drug sensitivity in non-small cell lung cancer. Pharmacol Rev. 2003;55:57–103. - PubMed
-
- Yang P, Allen MS, Aubry MC, Wampfler JA, Marks RS, et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128:452–462. - PubMed
-
- Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol. 2004;22:330–353. - PubMed
-
- Stinchcombe TE, Socinski MA. Current treatments for advanced stage non-small cell lung cancer. Proc Am Thorac Soc. 2009;6:233–241. - PubMed
-
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

