A new method for the characterization of strain-specific conformational stability of protease-sensitive and protease-resistant PrPSc
- PMID: 20856860
- PMCID: PMC2939050
- DOI: 10.1371/journal.pone.0012723
A new method for the characterization of strain-specific conformational stability of protease-sensitive and protease-resistant PrPSc
Abstract
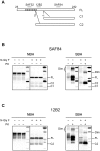
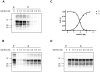
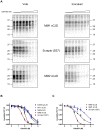
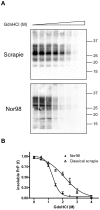
Although proteinacious in nature, prions exist as strains with specific self-perpetuating biological properties. Prion strains are thought to be associated with different conformers of PrP(Sc), a disease-associated isoform of the host-encoded cellular protein (PrP(C)). Molecular strain typing approaches have been developed which rely on the characterization of protease-resistant PrP(Sc). However, PrP(Sc) is composed not only of protease-resistant but also of protease-sensitive isoforms. The aim of this work was to develop a protocol for the molecular characterization of both, protease-resistant and protease-sensitive PrP(Sc) aggregates. We first set up experimental conditions which allowed the most advantageous separation of PrP(C) and PrP(Sc) by means of differential centrifugation. The conformational solubility and stability assay (CSSA) was then developed by measuring PrP(Sc) solubility as a function of increased exposure to GdnHCl. Brain homogenates from voles infected with human and sheep prion isolates were analysed by CSSA and showed strain-specific conformational stabilities, with mean [GdnHCl](1/2) values ranging from 1.6 M for MM2 sCJD to 2.1 for scrapie and to 2.8 M for MM1/MV1 sCJD and E200K gCJD. Interestingly, the rank order of [GdnHCl](1/2) values observed in the human and sheep isolates used as inocula closely matched those found following transmission in voles, being MM1 sCJD the most resistant (3.3 M), followed by sheep scrapie (2.2 M) and by MM2 sCJD (1.6 M). In order to test the ability of CSSA to characterise protease-sensitive PrP(Sc), we analysed sheep isolates of Nor98 and compared them to classical scrapie isolates. In Nor98, insoluble PrP(Sc) aggregates were mainly protease-sensitive and showed a conformational stability much lower than in classical scrapie. Our results show that CSSA is able to reveal strain-specified PrP(Sc) conformational stabilities of protease-resistant and protease-sensitive PrP(Sc) and that it is a valuable tool for strain typing in natural hosts, such as humans and sheep.
Conflict of interest statement
Figures



Similar articles
-
Protease-resistant prions selectively decrease Shadoo protein.PLoS Pathog. 2011 Nov;7(11):e1002382. doi: 10.1371/journal.ppat.1002382. Epub 2011 Nov 17. PLoS Pathog. 2011. PMID: 22163178 Free PMC article.
-
Analyses of protease resistance and aggregation state of abnormal prion protein across the spectrum of human prions.J Biol Chem. 2013 Sep 27;288(39):27972-85. doi: 10.1074/jbc.M113.477547. Epub 2013 Jul 29. J Biol Chem. 2013. PMID: 23897825 Free PMC article.
-
The Size and Stability of Infectious Prion Aggregates Fluctuate Dynamically during Cellular Uptake and Disaggregation.Biochemistry. 2021 Feb 9;60(5):398-411. doi: 10.1021/acs.biochem.0c00923. Epub 2021 Jan 26. Biochemistry. 2021. PMID: 33497187
-
Biochemistry and structure of PrP(C) and PrP(Sc).Br Med Bull. 2003;66:21-33. doi: 10.1093/bmb/66.1.21. Br Med Bull. 2003. PMID: 14522846 Review.
-
Molecular biology and pathology of prion strains in sporadic human prion diseases.Acta Neuropathol. 2011 Jan;121(1):79-90. doi: 10.1007/s00401-010-0761-3. Epub 2010 Nov 7. Acta Neuropathol. 2011. PMID: 21058033 Free PMC article. Review.
Cited by
-
Relationships between PrPSc stability and incubation time for United States scrapie isolates in a natural host system.PLoS One. 2012;7(8):e43060. doi: 10.1371/journal.pone.0043060. Epub 2012 Aug 14. PLoS One. 2012. PMID: 22916207 Free PMC article.
-
Recent advances in the histo-molecular pathology of human prion disease.Brain Pathol. 2019 Mar;29(2):278-300. doi: 10.1111/bpa.12695. Epub 2019 Jan 22. Brain Pathol. 2019. PMID: 30588685 Free PMC article. Review.
-
Prion Strain Characterization of a Novel Subtype of Creutzfeldt-Jakob Disease.J Virol. 2017 May 12;91(11):e02390-16. doi: 10.1128/JVI.02390-16. Print 2017 Jun 1. J Virol. 2017. PMID: 28298604 Free PMC article.
-
Stability properties of PrP(Sc) from cattle with experimental transmissible spongiform encephalopathies: use of a rapid whole homogenate, protease-free assay.BMC Vet Res. 2013 Aug 15;9:167. doi: 10.1186/1746-6148-9-167. BMC Vet Res. 2013. PMID: 23945217 Free PMC article.
-
Novel strain properties distinguishing sporadic prion diseases sharing prion protein genotype and prion type.Sci Rep. 2017 Jan 16;7:38280. doi: 10.1038/srep38280. Sci Rep. 2017. PMID: 28091514 Free PMC article.
References
-
- Benestad SL, Sarradin P, Thu B, Schonheit J, Tranulis MA, et al. Cases of scrapie with unusual features in Norway and designation of a new type, Nor98. Vet Rec. 2003;153:202–208. - PubMed
-
- Bolton DC, McKinley MP, Prusiner SB. Identification of a protein that purifies with the scrapie prion. Science. 1982;218:1309–1311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

