MnSOD upregulation induces autophagic programmed cell death in senescent keratinocytes
- PMID: 20856861
- PMCID: PMC2939051
- DOI: 10.1371/journal.pone.0012712
MnSOD upregulation induces autophagic programmed cell death in senescent keratinocytes
Abstract
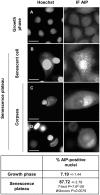
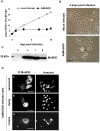
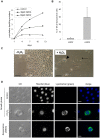
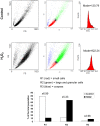
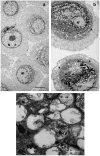
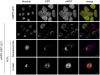
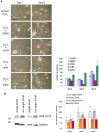
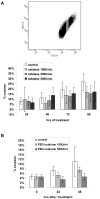
Senescence is a state of growth arrest resulting mainly from telomere attrition and oxidative stress. It ultimately leads to cell death. We have previously shown that, in keratinocytes, senescence is induced by NF-kappaB activation, MnSOD upregulation and H(2)O(2) overproduction. We have also shown that senescent keratinocytes do not die by apoptosis but as a result of high macroautophagic activity that targets the primary vital cell components. Here, we investigated the mechanisms that activate this autophagic cell death program. We show that corpses occurring at the senescence plateau display oxidatively-damaged mitochondria and nucleus that colocalize with autophagic vacuoles. The occurrence of such corpses was decreased by specifically reducing the H(2)O(2) level with catalase, and, conversely, reproduced by overexpressing MnSOD or applying subtoxic doses of H(2)O(2). This H(2)O(2)-induced cell death did occur through autophagy since it was accompanied by an accumulation of autophagic vesicles as evidenced by Lysotracker staining, LC3 vesiculation and transmission electron microscopy. Most importantly, it was partly abolished by 3-methyladenine, the specific inhibitor of autophagosome formation, and by anti-Atg5 siRNAs. Taken together these results suggest that autophagic cell death is activated in senescent keratinocytes because of the upregulation of MnSOD and the resulting accumulation of oxidative damages to nucleus and mitochondria.
Conflict of interest statement
Figures
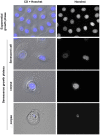
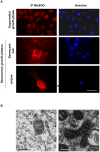
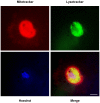
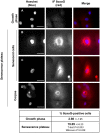
Similar articles
-
Level of macroautophagy drives senescent keratinocytes into cell death or neoplastic evasion.Cell Death Dis. 2014 Dec 18;5(12):e1577. doi: 10.1038/cddis.2014.533. Cell Death Dis. 2014. PMID: 25522271 Free PMC article.
-
Senescent keratinocytes die by autophagic programmed cell death.Am J Pathol. 2009 Feb;174(2):423-35. doi: 10.2353/ajpath.2009.080332. Epub 2009 Jan 15. Am J Pathol. 2009. PMID: 19147823 Free PMC article.
-
Involvement of Rel/nuclear factor-kappaB transcription factors in keratinocyte senescence.Cancer Res. 2004 Jan 15;64(2):472-81. doi: 10.1158/0008-5472.can-03-0005. Cancer Res. 2004. PMID: 14744759
-
cRel induces mitochondrial alterations in correlation with proliferation arrest.Free Radic Biol Med. 2001 Oct 15;31(8):943-53. doi: 10.1016/s0891-5849(01)00668-2. Free Radic Biol Med. 2001. PMID: 11595379
-
Timosaponin A-III induces autophagy preceding mitochondria-mediated apoptosis in HeLa cancer cells.Cancer Res. 2008 Dec 15;68(24):10229-37. doi: 10.1158/0008-5472.CAN-08-1983. Cancer Res. 2008. PMID: 19074891
Cited by
-
Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin.Prog Neurobiol. 2012 Nov;99(2):128-48. doi: 10.1016/j.pneurobio.2012.08.001. Epub 2012 Aug 15. Prog Neurobiol. 2012. PMID: 22980037 Free PMC article. Review.
-
Proteasome-mediated degradation of keratins 7, 8, 17 and 18 by mutant KLHL24 in a foetal keratinocyte model: Novel insight in congenital skin defects and fragility of epidermolysis bullosa simplex with cardiomyopathy.Hum Mol Genet. 2022 Apr 22;31(8):1308-1324. doi: 10.1093/hmg/ddab318. Hum Mol Genet. 2022. PMID: 34740256 Free PMC article.
-
Prostaglandin E2 regulates senescence and post-senescence neoplastic escape in primary human keratinocytes.Aging (Albany NY). 2024 Nov 18;16(21):13201-13224. doi: 10.18632/aging.206149. Epub 2024 Nov 18. Aging (Albany NY). 2024. PMID: 39560493 Free PMC article.
-
Consequences of Autophagy Deletion on the Age-Related Changes in the Epidermal Lipidome of Mice.Int J Mol Sci. 2022 Sep 21;23(19):11110. doi: 10.3390/ijms231911110. Int J Mol Sci. 2022. PMID: 36232414 Free PMC article.
-
Autophagy and senescence: a partnership in search of definition.Autophagy. 2013 May;9(5):808-12. doi: 10.4161/auto.23922. Epub 2013 Feb 19. Autophagy. 2013. PMID: 23422284 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

