Colonic biopsies to assess the neuropathology of Parkinson's disease and its relationship with symptoms
- PMID: 20856865
- PMCID: PMC2939055
- DOI: 10.1371/journal.pone.0012728
Colonic biopsies to assess the neuropathology of Parkinson's disease and its relationship with symptoms
Abstract
Background: The presence of Lewy bodies and Lewy neurites (LN) has been demonstrated in the enteric nervous system (ENS) of Parkinson's disease (PD) patients. The aims of the present research were to use routine colonoscopy biopsies (1) to analyze, in depth, enteric pathology throughout the colonic submucosal plexus (SMP), and (2) to correlate the pathological burden with neurological and gastrointestinal (GI) symptoms.
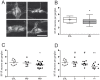
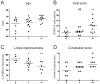
Methodology/principal findings: A total of 10 control and 29 PD patients divided into 3 groups according to disease duration were included. PD and GI symptoms were assessed using the Unified Parkinson's Disease Rating Scale part III and the Rome III questionnaire, respectively. Four biopsies were taken from the ascending and descending colon during the course of a total colonoscopy. Immunohistochemical analysis was performed using antibodies against phosphorylated alpha-synuclein, neurofilaments NF 220 kDa (NF) and tyrosine hydroxylase (TH). The density of LN, labeled by anti-phosphorylated alpha-synuclein antibodies, was evaluated using a quantitative rating score. Lewy pathology was apparent in the colonic biopsies from 21 patients and in none of the controls. A decreased number of NF-immunoreactive neurons per ganglion was observed in the SMP of PD patients compared to controls. The amount of LN in the ENS was inversely correlated with neuronal count and positively correlated with levodopa-unresponsive features and constipation.
Conclusion/significance: Analysis of the ENS by routine colonoscopy biopsies is a useful tool for pre-mortem neuropathological diagnosis of PD, and also provides insight into the progression of motor and non-motor symptoms.
Trial registration: ClinicalTrials.gov NCT00491062.
Conflict of interest statement
Figures

References
-
- Furness JB. The enteric nervous system: normal functions and enteric neuropathies. Neurogastroenterol Motil. 2008;20(Suppl 1):32–38. - PubMed
-
- De Giorgio R, Camilleri M. Human enteric neuropathies: morphology and molecular pathology. Neurogastroenterol Motil. 2004;16:515–531. - PubMed
-
- Basilisco G, Gebbia C, Peracchi M, Velio P, Conte D, et al. Cerebellar degeneration and hearing loss in a patient with idiopathic myenteric ganglionitis. Eur J Gastroenterol Hepatol. 2005;17:449–452. - PubMed
-
- Haik S, Faucheux BA, Hauw JJ. Brain targeting through the autonomous nervous system: lessons from prion diseases. Trends Mol Med. 2004;10:107–112. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

