Prohibitins are required for cancer cell proliferation and adhesion
- PMID: 20856874
- PMCID: PMC2939069
- DOI: 10.1371/journal.pone.0012735
Prohibitins are required for cancer cell proliferation and adhesion
Abstract
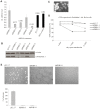
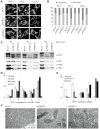
Prohibitin 1 (PHB1) is a highly conserved protein that together with its homologue prohibitin 2 (PHB2) mainly localizes to the inner mitochondrial membrane. Although it was originally identified by its ability to inhibit G1/S progression in human fibroblasts, its role as tumor suppressor is debated. To determine the function of prohibitins in maintaining cell homeostasis, we generated cancer cell lines expressing prohibitin-directed shRNAs. We show that prohibitin proteins are necessary for the proliferation of cancer cells. Down-regulation of prohibitin expression drastically reduced the rate of cell division. Furthermore, mitochondrial morphology was not affected, but loss of prohibitins did lead to the degradation of the fusion protein OPA1 and, in certain cancer cell lines, to a reduced capability to exhibit anchorage-independent growth. These cancer cells also exhibited reduced adhesion to the extracellular matrix. Taken together, these observations suggest prohibitins play a crucial role in adhesion processes in the cell and thereby sustaining cancer cell propagation and survival.
Conflict of interest statement
Figures


References
-
- McClung JK, Jupe ER, Liu XT, Dell'Orco RT. Prohibitin: potential role in senescence, development, and tumor suppression. Exp Gerontol. 1995;30:99–124. - PubMed
-
- Wang S, Fusaro G, Padmanabhan J, Chellappan SP. Prohibitin co-localizes with Rb in the nucleus and recruits N-CoR and HDAC1 for transcriptional repression. Oncogene. 2002;21:8388–8396. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

