MPF governs the assembly and contraction of actomyosin rings by activating RhoA and MAPK during chemical-induced cytokinesis of goat oocytes
- PMID: 20856880
- PMCID: PMC2938347
- DOI: 10.1371/journal.pone.0012706
MPF governs the assembly and contraction of actomyosin rings by activating RhoA and MAPK during chemical-induced cytokinesis of goat oocytes
Abstract
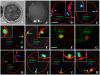
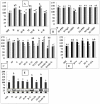
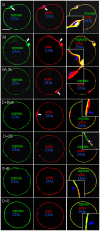
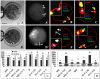
The interplay between maturation-promoting factor (MPF), mitogen-activated protein kinase (MAPK) and Rho GTPase during actin-myosin interactions has yet to be determined. The mechanism by which microtubule disrupters induce the formation of ooplasmic protrusion during chemical-assisted enucleation of mammalian oocytes is unknown. Moreover, a suitable model is urgently needed for the study of cytokinesis. We have established a model of chemical-induced cytokinesis and have studied the signaling events leading to cytokinesis using this model. The results suggested that microtubule inhibitors activated MPF, which induced actomyosin assembly (formation of ooplasmic protrusion) by activating RhoA and thus MAPK. While MAPK controlled actin recruitment on its own, MPF promoted myosin enrichment by activating RhoA and MAPK. A further chemical treatment of oocytes with protrusions induced constriction of the actomyosin ring by inactivating MPF while activating RhoA. In conclusion, the present data suggested that the assembly and contraction of the actomyosin ring were two separable steps: while an increase in MPF activity promoted the assembly through RhoA-mediated activation of MAPK, a decrease in MPF activity triggered contraction of the ring by activating RhoA.
Conflict of interest statement
Figures

Similar articles
-
Regulation of fusion of the nucleolar precursor bodies following activation of mouse oocytes: roles of the maturation-promoting factors and mitogen-activated protein kinases.Zygote. 2012 Aug;20(3):291-303. doi: 10.1017/S0967199411000219. Epub 2011 May 4. Zygote. 2012. PMID: 21554769
-
Discrimination of the roles of MPF and MAP kinase in morphological changes that occur during oocyte maturation.Dev Biol. 2002 Dec 15;252(2):271-86. doi: 10.1006/dbio.2002.0853. Dev Biol. 2002. PMID: 12482715
-
Small GTPase RhoA regulates cytoskeleton dynamics during porcine oocyte maturation and early embryo development.Cell Cycle. 2014;13(21):3390-403. doi: 10.4161/15384101.2014.952967. Cell Cycle. 2014. PMID: 25485583 Free PMC article.
-
Shared mechanisms regulate spatiotemporal RhoA-dependent actomyosin contractility during adhesion and cell division.Small GTPases. 2020 Mar;11(2):113-121. doi: 10.1080/21541248.2017.1366966. Epub 2017 Dec 31. Small GTPases. 2020. PMID: 29291271 Free PMC article. Review.
-
FilGAP and its close relatives: a mediator of Rho-Rac antagonism that regulates cell morphology and migration.Biochem J. 2013 Jul 1;453(1):17-25. doi: 10.1042/BJ20130290. Biochem J. 2013. PMID: 23763313 Review.
Cited by
-
Effects of Short-Term Inhibition of Rho Kinase on Dromedary Camel Oocyte In Vitro Maturation.Animals (Basel). 2020 Apr 25;10(5):750. doi: 10.3390/ani10050750. Animals (Basel). 2020. PMID: 32344840 Free PMC article.
-
Handmade cloning: recent advances, potential and pitfalls.J Anim Sci Biotechnol. 2015 Oct 15;6:43. doi: 10.1186/s40104-015-0043-y. eCollection 2015. J Anim Sci Biotechnol. 2015. PMID: 26473031 Free PMC article. Review.
-
Effect of demecolcine-assisted enucleation on the MPF level and cyclin B1 distribution in porcine oocytes.PLoS One. 2014 Mar 13;9(3):e91483. doi: 10.1371/journal.pone.0091483. eCollection 2014. PLoS One. 2014. PMID: 24626152 Free PMC article.
References
-
- Barr FA, Gruneberg U. Cytokinesis: placing and making the final cut. Cell. 2007;131:847–860. - PubMed
-
- Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol. 2002;4:294–301. - PubMed
-
- Mishima M, Glotzer M. Cytokinesis. In: Lennarz WJ and Lane MD (eds) “Encyclopedia of Biological Chemistry”, Elsevier Inc. Volume. 2004;1:556–561.
-
- Yamashiro S, Yamakita Y, Hosoya H, Matsumura F. Phosphorylation of non-muscle caldesmon by p34cdc2 kinase during mitosis. Nature. 1991;349:169–172. - PubMed
-
- Samaj J, Baluska F, Hirt H. From signal to cell polarity: mitogen-activated protein kinases as sensors and effectors of cytoskeleton dynamicity. J Exp Bot. 2004;55:189–198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

