Renal Dnase1 enzyme activity and protein expression is selectively shut down in murine and human membranoproliferative lupus nephritis
- PMID: 20856893
- PMCID: PMC2938370
- DOI: 10.1371/journal.pone.0012096
Renal Dnase1 enzyme activity and protein expression is selectively shut down in murine and human membranoproliferative lupus nephritis
Abstract
Background: Deposition of chromatin-IgG complexes within glomerular membranes is a key event in the pathogenesis of lupus nephritis. We recently reported an acquired loss of renal Dnase1 expression linked to transformation from mild to severe membranoproliferative lupus nephritis in (NZBxNZW)F1 mice. As this may represent a basic mechanism in the progression of lupus nephritis, several aspects of Dnase1 expression in lupus nephritis were analyzed.
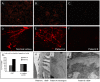
Methodology/principal findings: Total nuclease activity and Dnase1 expression and activity was evaluated using in situ and in vitro analyses of kidneys and sera from (NZBxNZW)F1 mice of different ages, and from age-matched healthy controls. Immunofluorescence staining for Dnase1 was performed on kidney biopsies from (NZBxNZW)F1 mice as well as from human SLE patients and controls. Reduced serum Dnase1 activity was observed in both mesangial and end-stage lupus nephritis. A selective reduction in renal Dnase1 activity was seen in mice with massive deposition of chromatin-containing immune complexes in glomerular capillary walls. Mice with mild mesangial nephritis showed normal renal Dnase1 activity. Similar differences were seen when comparing human kidneys with severe and mild lupus nephritis. Dnase1 was diffusely expressed within the kidney in normal and mildly affected kidneys, whereas upon progression towards end-stage renal disease, Dnase1 was down-regulated in all renal compartments. This demonstrates that the changes associated with development of severe nephritis in the murine model are also relevant to human lupus nephritis.
Conclusions/significance: Reduction in renal Dnase1 expression and activity is limited to mice and SLE patients with signs of membranoproliferative nephritis, and may be a critical event in the development of severe forms of lupus nephritis. Reduced Dnase1 activity reflects loss in the expression of the protein and not inhibition of enzyme activity.
Conflict of interest statement
Figures

Similar articles
-
Anti-dsDNA antibodies promote initiation, and acquired loss of renal Dnase1 promotes progression of lupus nephritis in autoimmune (NZBxNZW)F1 mice.PLoS One. 2009 Dec 29;4(12):e8474. doi: 10.1371/journal.pone.0008474. PLoS One. 2009. PMID: 20041189 Free PMC article.
-
Progression of murine lupus nephritis is linked to acquired renal Dnase1 deficiency and not to up-regulated apoptosis.Am J Pathol. 2009 Jul;175(1):97-106. doi: 10.2353/ajpath.2009.080943. Epub 2009 Jun 15. Am J Pathol. 2009. PMID: 19528352 Free PMC article.
-
Acquired loss of renal nuclease activity is restricted to DNaseI and is an organ-selective feature in murine lupus nephritis.Am J Pathol. 2011 Sep;179(3):1120-8. doi: 10.1016/j.ajpath.2011.05.011. Epub 2011 Jun 30. Am J Pathol. 2011. PMID: 21723244 Free PMC article.
-
Lupus nephritis: enigmas, conflicting models and an emerging concept.Mol Med. 2013 Jul 24;19(1):161-9. doi: 10.2119/molmed.2013.00010. Mol Med. 2013. PMID: 23752208 Free PMC article. Review.
-
Nuclease deficiencies promote end-stage lupus nephritis but not nephritogenic autoimmunity in (NZB × NZW) F1 mice.Immunol Cell Biol. 2011 Jan;89(1):90-9. doi: 10.1038/icb.2010.75. Epub 2010 Jun 15. Immunol Cell Biol. 2011. PMID: 20548325 Review.
Cited by
-
Comparative transcriptional profiling of 3 murine models of SLE nephritis reveals both unique and shared regulatory networks.PLoS One. 2013 Oct 22;8(10):e77489. doi: 10.1371/journal.pone.0077489. eCollection 2013. PLoS One. 2013. PMID: 24167575 Free PMC article.
-
The Role of Nucleases and Nucleic Acid Editing Enzymes in the Regulation of Self-Nucleic Acid Sensing.Front Immunol. 2021 Feb 26;12:629922. doi: 10.3389/fimmu.2021.629922. eCollection 2021. Front Immunol. 2021. PMID: 33717156 Free PMC article. Review.
-
The origin and properties of extracellular DNA: from PAMP to DAMP.Clin Immunol. 2012 Jul;144(1):32-40. doi: 10.1016/j.clim.2012.04.006. Epub 2012 May 3. Clin Immunol. 2012. PMID: 22659033 Free PMC article. Review.
-
Neutrophil extracellular chromatin traps connect innate immune response to autoimmunity.Semin Immunopathol. 2013 Jul;35(4):465-80. doi: 10.1007/s00281-013-0376-6. Epub 2013 Apr 18. Semin Immunopathol. 2013. PMID: 23595413 Review.
-
Pathogenesis of autoimmune disease.Nat Rev Nephrol. 2023 Aug;19(8):509-524. doi: 10.1038/s41581-023-00720-1. Epub 2023 May 10. Nat Rev Nephrol. 2023. PMID: 37165096 Free PMC article. Review.
References
-
- Miescher P, Fauconnet M. Absorption of L. E. factor by isolated cell nuclei. Experientia. 1954;10:252–253. - PubMed
-
- Hahn BH. Antibodies to DNA. N Engl J Med. 1998;338:1359–1368. - PubMed
-
- Mortensen ES, Rekvig OP. Nephritogenic potential of anti-DNA antibodies against necrotic nucleosomes. J Am Soc Nephrol. 2009;20:696–704. - PubMed
-
- Gaipl US, Kuhn A, Sheriff A, Munoz LE, Franz S, et al. Clearance of apoptotic cells in human SLE. Curr Dir Autoimmun. 2006;9:173–187. - PubMed
-
- Gaipl US, Sheriff A, Franz S, Munoz LE, Voll RE, et al. Inefficient clearance of dying cells and autoreactivity. Curr Top Microbiol Immunol. 2006;305:161–176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

