Lethality and developmental delay in Drosophila melanogaster larvae after ingestion of selected Pseudomonas fluorescens strains
- PMID: 20856932
- PMCID: PMC2938339
- DOI: 10.1371/journal.pone.0012504
Lethality and developmental delay in Drosophila melanogaster larvae after ingestion of selected Pseudomonas fluorescens strains
Abstract
Background: The fruit fly, Drosophila melanogaster, is a well-established model organism for probing the molecular and cellular basis of physiological and immune system responses of adults or late stage larvae to bacterial challenge. However, very little is known about the consequences of bacterial infections that occur in earlier stages of development. We have infected mid-second instar larvae with strains of Pseudomonas fluorescens to determine how infection alters the ability of larvae to survive and complete development.
Methodology/principal findings: We mimicked natural routes of infection using a non-invasive feeding procedure to study the toxicity of the three sequenced P. fluorescens strains (Pf0-1, SBW25, and Pf-5) to Drosophila melanogaster. Larvae fed with the three strains of P. fluorescens showed distinct differences in developmental trajectory and survival. Treatment with SBW25 caused a subset of insects to die concomitant with a systemic melanization reaction at larval, pupal or adult stages. Larvae fed with Pf-5 died in a dose-dependent manner with adult survivors showing eye and wing morphological defects. In addition, larvae in the Pf-5 treatment groups showed a dose-dependent delay in the onset of metamorphosis relative to control-, Pf0-1-, and SBW25-treated larvae. A functional gacA gene is required for the toxic properties of wild-type Pf-5 bacteria.
Conclusions/significance: These experiments are the first to demonstrate that ingestion of P. fluorescens bacteria by D. melanogaster larvae causes both lethal and non-lethal phenotypes, including delay in the onset of metamorphosis and morphological defects in surviving adult flies, which can be decoupled.
Conflict of interest statement
Figures

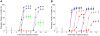
 ; SBW25,
; SBW25,  ; Pf-5,
; Pf-5,  , or (B) Pf0-1,
, or (B) Pf0-1,  ; Pf-5 gacA mutant,
; Pf-5 gacA mutant,  ; Pf-5 (squares),
; Pf-5 (squares),  ; Pf-5 (triangles),
; Pf-5 (triangles),  .
.
 cfu/plate, gray circles) or control (black circles ) treatment were determined for each time point. The Pf-5 cell densities as cfu/plate were
cfu/plate, gray circles) or control (black circles ) treatment were determined for each time point. The Pf-5 cell densities as cfu/plate were  (circles),
(circles),  (squares),
(squares),  (diamonds),
(diamonds),  (triangles),
(triangles),  (stars) cfu/plate.
(stars) cfu/plate.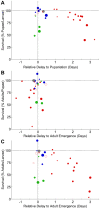
 cfu/plate; smallest dose/smallest circle is
cfu/plate; smallest dose/smallest circle is  cfu/plate; highest dose/largest diamond is
cfu/plate; highest dose/largest diamond is  ; lowest dose/smallest diamond is
; lowest dose/smallest diamond is  ); Pf0-1 (blue), SBW25 (green), Pf-5 (red), killed Pf-5 (gray) and a Pf-5 gacA mutant (open red circle). (A) The relative developmental delay (see Materials and Methods) during the larval stage (X-axis) was plotted against the percentage of larvae that survived to pupariate (Y-axis) for six different experiments. (B) The relative developmental delay during the pupal stage (X-axis) was plotted against the percentage of pupae that survived to adult survivors (Y-axis). (C) The relative developmental delay during larval and pupal stages (X-axis) was plotted against the percentage of larvae that survived to adult (Y-axis).
); Pf0-1 (blue), SBW25 (green), Pf-5 (red), killed Pf-5 (gray) and a Pf-5 gacA mutant (open red circle). (A) The relative developmental delay (see Materials and Methods) during the larval stage (X-axis) was plotted against the percentage of larvae that survived to pupariate (Y-axis) for six different experiments. (B) The relative developmental delay during the pupal stage (X-axis) was plotted against the percentage of pupae that survived to adult survivors (Y-axis). (C) The relative developmental delay during larval and pupal stages (X-axis) was plotted against the percentage of larvae that survived to adult (Y-axis).
 cfu/plate). (C) 72 hour post Pf-5 gacA mutant treatment (
cfu/plate). (C) 72 hour post Pf-5 gacA mutant treatment ( cfu/plate). (D) 48 hour post Pf-5 treatment (
cfu/plate). (D) 48 hour post Pf-5 treatment ( cfu/plate), showing small body size and convoluted longitudinal tracheal trunks. (E) 72 hour post Pf-5 treatment (
cfu/plate), showing small body size and convoluted longitudinal tracheal trunks. (E) 72 hour post Pf-5 treatment ( cfu/plate), showing small body size and convoluted tracheal trunks. (F) 72 hour post Pf-5 treatment (
cfu/plate), showing small body size and convoluted tracheal trunks. (F) 72 hour post Pf-5 treatment ( cfu/plate), showing loss of fat body opacity and a melanotic nodule. (G) 72 hour post SBW25 treatment (2.9×107 cfu/plate), with no melanization within the body cavity. (H) 72 hour post SBW25 treatment (
cfu/plate), showing loss of fat body opacity and a melanotic nodule. (G) 72 hour post SBW25 treatment (2.9×107 cfu/plate), with no melanization within the body cavity. (H) 72 hour post SBW25 treatment ( cfu/plate) showing melanization within the posterior third of the body. (I) 72 hour post SBW25 treatment (
cfu/plate) showing melanization within the posterior third of the body. (I) 72 hour post SBW25 treatment ( cfu/plate) showing complete melanization reaction.
cfu/plate) showing complete melanization reaction.
 cfu/plate, Pf0-1;
cfu/plate, Pf0-1;  cfu/plate Pf-5 gacA mutant;
cfu/plate Pf-5 gacA mutant;  cfu/plate killed Pf-5. Bottom panel from left to right:
cfu/plate killed Pf-5. Bottom panel from left to right:  cfu/plate Pf-5;
cfu/plate Pf-5;  cfu/plate Pf-5;
cfu/plate Pf-5;  cfu/plate Pf-5;
cfu/plate Pf-5;  cfu/plate SBW25. Magnification bar = 500 µm. (B) Images of wing blades from adult flies after control (upper left) or Pf-5 at
cfu/plate SBW25. Magnification bar = 500 µm. (B) Images of wing blades from adult flies after control (upper left) or Pf-5 at  cfu/plate (bottom left, right panel) treatments. Developmental defects visible in these wings include loss of cells at the distal margin (upper right) and/or the presence of ectopic wing veins (bottom left and right). Magnification bar = 100 µm.
cfu/plate (bottom left, right panel) treatments. Developmental defects visible in these wings include loss of cells at the distal margin (upper right) and/or the presence of ectopic wing veins (bottom left and right). Magnification bar = 100 µm.
Similar articles
-
A critical role for the Drosophila dopamine D1-like receptor Dop1R2 at the onset of metamorphosis.BMC Dev Biol. 2016 May 16;16(1):15. doi: 10.1186/s12861-016-0115-z. BMC Dev Biol. 2016. PMID: 27184815 Free PMC article.
-
Sensitivity differences displayed by Drosophila melanogaster larvae of different ages to the toxic effects of growth on media containing aflatoxin B1.Chem Biol Interact. 1979 Mar;24(3):373-80. doi: 10.1016/0009-2797(79)90084-x. Chem Biol Interact. 1979. PMID: 106976
-
Suppressed production of methyl farnesoid hormones yields developmental defects and lethality in Drosophila larvae.Gen Comp Endocrinol. 2010 Jan 15;165(2):244-54. doi: 10.1016/j.ygcen.2009.07.006. Epub 2009 Jul 10. Gen Comp Endocrinol. 2010. PMID: 19595690 Free PMC article.
-
Persistence of an extracellular systemic infection across metamorphosis in a holometabolous insect.Biol Lett. 2018 Feb;14(2):20170771. doi: 10.1098/rsbl.2017.0771. Biol Lett. 2018. PMID: 29438055 Free PMC article.
-
Insect hemolymph clotting.Cell Mol Life Sci. 2009 Aug;66(16):2643-50. doi: 10.1007/s00018-009-0036-0. Epub 2009 May 6. Cell Mol Life Sci. 2009. PMID: 19418022 Free PMC article. Review.
Cited by
-
Drosophila melanogaster larvae are tolerant to oral infection with the bacterial pathogen Photorhabdus luminescens.MicroPubl Biol. 2023 Aug 29;2023:10.17912/micropub.biology.000938. doi: 10.17912/micropub.biology.000938. eCollection 2023. MicroPubl Biol. 2023. PMID: 37711508 Free PMC article.
-
Isolation, identification and pathogenicity of local entomopathogenic bacteria as biological control agents against the wild cochineal Dactylopius opuntiae (Cockerell) on cactus pear in Morocco.Sci Rep. 2023 Dec 8;13(1):21647. doi: 10.1038/s41598-023-48976-8. Sci Rep. 2023. PMID: 38062128 Free PMC article.
-
Comparative genomics of plant-associated Pseudomonas spp.: insights into diversity and inheritance of traits involved in multitrophic interactions.PLoS Genet. 2012 Jul;8(7):e1002784. doi: 10.1371/journal.pgen.1002784. Epub 2012 Jul 5. PLoS Genet. 2012. PMID: 22792073 Free PMC article.
-
The secret life of plant-beneficial rhizosphere bacteria: insects as alternative hosts.Environ Microbiol. 2022 Aug;24(8):3273-3289. doi: 10.1111/1462-2920.15968. Epub 2022 Mar 26. Environ Microbiol. 2022. PMID: 35315557 Free PMC article. Review.
-
In vivo Toxicity Assessment of Antimicrobial Peptides (AMPs LR14) Derived from Lactobacillus plantarum Strain LR/14 in Drosophila melanogaster.Probiotics Antimicrob Proteins. 2014 Mar;6(1):59-67. doi: 10.1007/s12602-013-9154-y. Probiotics Antimicrob Proteins. 2014. PMID: 24676768
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

