Inhibition of melanoma growth by subcutaneous administration of hTERTC27 viral cocktail in C57BL/6 mice
- PMID: 20856939
- PMCID: PMC2938346
- DOI: 10.1371/journal.pone.0012705
Inhibition of melanoma growth by subcutaneous administration of hTERTC27 viral cocktail in C57BL/6 mice
Abstract
Background: hTERTC27 is a 27 kDa C-terminal polypeptide of human telomerase reverse transcriptase that has previously been shown to reduce tumorigenicity of HeLa cells and suppress growth of xenografted glioblastoma in nude mice. Although ectopic expression of hTERTC27 upregulated genes that are involved in apoptosis, cell cycle, and immune response, the mechanism for hTERTC27-induced tumor suppression has not been completely elucidated. Since hTERT was identified as a universal tumor-associated antigen, we hypothesize that hTERTC27 inhibits tumor growth in vivo through activation of anti-tumor immune response.
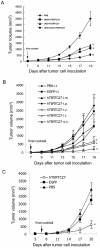
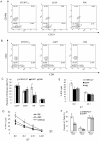
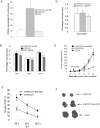
Methodology/principal finding: Immunocompetent C57BL/6 mice were used for mouse B16 melanoma model. Mice bearing B16 melanoma were administered rAAV-/rAdv viral cocktail expressing hTERTC27, and tumor growth was monitored after viral cocktail treatment. Blood and splenocytes were used to determine the level of cytokines and the activity of immune cells, respectively. B16 tumor growth was significantly inhibited by subcutaneous administration of a single dose of 1.5×10(11) vg rAAV-hTERTC27 and 2.5×10(9) pfu rAdv-hTERTC27 viral cocktail (rAAV-/rAdv-hTERTC27). The population and cytotoxicity of NK cells in the mice were significantly augmented by rAAV-/rAdv-hTERTC27 treatment, and selective depletion of the NK cell population in mice by intraperitoneal injection of anti-GM1 antibody abrogated the growth suppression of melanoma induced by rAAV-/rAdv-hTERTC27 administration.
Conclusion: Activation of NK cells by administration of rAAV-/rAdv-hTERTC27 is critical for growth suppression of melanoma in mouse model.
Conflict of interest statement
Figures
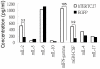
Similar articles
-
Suppressing tumor growth of nasopharyngeal carcinoma by hTERTC27 polypeptide delivered through adeno-associated virus plus adenovirus vector cocktail.Chin J Cancer. 2012 Dec;31(12):588-97. doi: 10.5732/cjc.011.10378. Epub 2012 Nov 13. Chin J Cancer. 2012. PMID: 23149313 Free PMC article.
-
Development of recombinant adeno-associated virus and adenovirus cocktail system for efficient hTERTC27 polypeptide-mediated cancer gene therapy.Cancer Gene Ther. 2008 Nov;15(11):723-32. doi: 10.1038/cgt.2008.33. Epub 2008 Jun 6. Cancer Gene Ther. 2008. PMID: 18535618
-
Inhibition of hepatocellular carcinoma growth by adenovirus-mediated expression of human telomerase reverse transcriptase COOH-27 terminal polypeptide in mice.Oncol Lett. 2013 Sep;6(3):748-752. doi: 10.3892/ol.2013.1470. Epub 2013 Jul 16. Oncol Lett. 2013. PMID: 24137404 Free PMC article.
-
A novel glioblastoma cancer gene therapy using AAV-mediated long-term expression of human TERT C-terminal polypeptide.Cancer Gene Ther. 2007 Jun;14(6):561-72. doi: 10.1038/sj.cgt.7701038. Epub 2007 Mar 23. Cancer Gene Ther. 2007. PMID: 17384579
-
Effective antitumor immunity against murine gliomas using dendritic cells transduced with hTERTC27 recombinant adenovirus.Oncol Rep. 2012 Apr;27(4):1163-9. doi: 10.3892/or.2011.1619. Epub 2011 Dec 30. Oncol Rep. 2012. PMID: 22210010 Free PMC article.
Cited by
-
Yessotoxin, a Marine Toxin, Exhibits Anti-Allergic and Anti-Tumoural Activities Inhibiting Melanoma Tumour Growth in a Preclinical Model.PLoS One. 2016 Dec 14;11(12):e0167572. doi: 10.1371/journal.pone.0167572. eCollection 2016. PLoS One. 2016. PMID: 27973568 Free PMC article.
-
The anti-hepatocellular carcinoma activity of Mel-P15 is mediated by natural killer cells.Oncol Lett. 2017 Dec;14(6):6901-6906. doi: 10.3892/ol.2017.7018. Epub 2017 Sep 21. Oncol Lett. 2017. PMID: 29163709 Free PMC article.
-
Targeting Telomere Dynamics as an Effective Approach for the Development of Cancer Therapeutics.Int J Nanomedicine. 2024 Apr 29;19:3805-3825. doi: 10.2147/IJN.S448556. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38708177 Free PMC article. Review.
-
In Vitro and In Vivo Tumor Growth Inhibition by Glutathione Disulfide Liposomes.Cancer Growth Metastasis. 2017 Mar 10;10:1179064417696070. doi: 10.1177/1179064417696070. eCollection 2017. Cancer Growth Metastasis. 2017. PMID: 28469472 Free PMC article.
-
Suppressing tumor growth of nasopharyngeal carcinoma by hTERTC27 polypeptide delivered through adeno-associated virus plus adenovirus vector cocktail.Chin J Cancer. 2012 Dec;31(12):588-97. doi: 10.5732/cjc.011.10378. Epub 2012 Nov 13. Chin J Cancer. 2012. PMID: 23149313 Free PMC article.
References
-
- Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma in the era of molecular profiling. Lancet. 2009;374:362–365. - PubMed
-
- Pawlik TM, Sondak VK. Malignant melanoma: current state of primary and adjuvant treatment. Crit Rev Oncol Hematol. 2003;45:245–264. - PubMed
-
- Eggermont AM, Kirkwood JM. Re-evaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years? Eur J Cancer. 2004;40:1825–1836. - PubMed
-
- Brown CK, Kirkwood JM. Medical management of melanoma. Surg Clin North Am. 2003;83:283–322, viii. - PubMed
-
- Flaherty LE, Atkins M, Sosman J, Weiss G, Clark JI, et al. Outpatient biochemotherapy with interleukin-2 and interferon alfa-2b in patients with metastatic malignant melanoma: results of two phase II cytokine working group trials. J Clin Oncol. 2001;19:3194–3202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

