Tetrathiafulvalene diindolylquinoxaline: a dual signaling anion receptor with phosphate selectivity
- PMID: 20856940
- PMCID: PMC3123379
- DOI: 10.1039/c0cc02934c
Tetrathiafulvalene diindolylquinoxaline: a dual signaling anion receptor with phosphate selectivity
Abstract
Incorporation of tetrathiafulvalene into the backbone of a known neutral phosphate receptor, diindolylquinoxaline, yields a dual optical-electrochemical chemosensor for dihydrogen phosphate that functions in dichloromethane. This system shows selectivity for dihydrogen phosphate over other small anions and can be used to detect the presence of this analyte via fluorescence quenching or cyclic voltammetry.
Figures

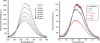

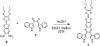
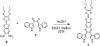
Similar articles
-
Diamino-diamido tetrathiafulvalene for the sensing of anions and cations: a view in electrochemistry and structure.J Phys Chem B. 2011 Mar 31;115(12):3020-6. doi: 10.1021/jp110010z. Epub 2011 Mar 8. J Phys Chem B. 2011. PMID: 21384812
-
Diindolylquinoxalines: effective indole-based receptors for phosphate anion.J Am Chem Soc. 2006 Dec 27;128(51):16518-9. doi: 10.1021/ja067720b. J Am Chem Soc. 2006. PMID: 17177398 Free PMC article.
-
A novel multisignaling optical-electrochemical chemosensor for anions based on tetrathiafulvalene.Org Lett. 2005 Oct 13;7(21):4629-32. doi: 10.1021/ol051735h. Org Lett. 2005. PMID: 16209496
-
Supported bilayer lipid membranes modified with a phosphate ionophore.Biosens Bioelectron. 2006 Jun 15;21(12):2311-4. doi: 10.1016/j.bios.2005.10.023. Epub 2005 Dec 1. Biosens Bioelectron. 2006. PMID: 16325385
-
[How have we found any new reactions in the field of the heterocyclic chemistry?].Yakugaku Zasshi. 2004 Apr;124(4):165-81. doi: 10.1248/yakushi.124.165. Yakugaku Zasshi. 2004. PMID: 15067182 Review. Japanese.
References
-
- Sessler JL, Katayev EA, Ustynyuk YA. Coord. Chem. Rev. 2006;250:3004.
-
- Beer PD, Gale PA. Angew. Chem., Int. Ed. 2001;40:486. - PubMed
-
- Antonisse MMG, Reinhoudt DN. Chem. Commun. 1998:443.
- Yoon J, Kim SK, Singh NJ, Lee JW, Yang YJ, Chellappan K, Kim KS. J. Org. Chem. 2004;69:581. - PubMed
- Kendo SI, Hiraoka Y, Kurumatani N, Yano Y. Chem. Commun. 2005:1720. - PubMed
- Caltagirone C, Gale PA, Hiscock JR, Brooks SJ, Hursthouse MB, Light ME. Chem. Commun. 2008:3007. - PubMed
- Tobey SL, Ansyln EV. Org. Lett. 2003;5:1821. - PubMed
- Beer PD, Dickson CAP, Fletcher N, Goulden AJ, Grieve A, Hodacova J, Wear T. J. Chem. Soc., Chem. Commun. 1993:828.
- Sessler JL, Plitt P, Gross DE, Lynch VM. Chem.–Eur. J. 2007;13:1374. - PubMed
- Kuo LJ, Liao JH, Chen CT, Huang CH, Chen CS, Fang JM. Org. Lett. 2003;5:1821. - PubMed
- Mateus P, Delgado R, Brando P, Flix V. J. Org. Chem. 2009;74:8638. - PubMed
- Katayev EA, Sessler JL, Khrustalev VN, Ustynyuk YA. J. Org. Chem. 2007;72:7244. - PubMed
-
- Yamada J-I, Sugimoto T. TTF Chemistry. Kodansha Ltd.; Springer-Verlag; Heidelberg; Tokyo: Berlin: New York:
- Canevet D, Sallé M, Zhang G, Zhang D, Zhub D. Chem. Commun. 2009:2225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

