Definition of treatment goals for moderate to severe psoriasis: a European consensus
- PMID: 20857129
- PMCID: PMC3016217
- DOI: 10.1007/s00403-010-1080-1
Definition of treatment goals for moderate to severe psoriasis: a European consensus
Abstract
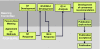
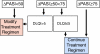
Patients with moderate to severe psoriasis are undertreated. To solve this persistent problem, the consensus programme was performed to define goals for treatment of plaque psoriasis with systemic therapy and to improve patient care. An expert consensus meeting and a collaborative Delphi procedure were carried out. Nineteen dermatologists from different European countries met for a face-to-face discussion and defined items through a four-round Delphi process. Severity of plaque psoriasis was graded into mild and moderate to severe disease. Mild disease was defined as body surface area (BSA) ≤10 and psoriasis area and severity index (PASI) ≤10 and dermatology life quality index (DLQI) ≤10 and moderate to severe psoriasis as (BSA > 10 or PASI > 10) and DLQI > 10. Special clinical situations may change mild psoriasis to moderate to severe including involvement of visible areas or severe nail involvement. For systemic therapy of plaque psoriasis two treatment phases were defined: (1) induction phase as the treatment period until week 16; however, depending on the type of drug and dose regimen used, this phase may be extended until week 24 and (2) maintenance phase for all drugs was defined as the treatment period after the induction phase. For the definition of treatment goals in plaque psoriasis, the change of PASI from baseline until the time of evaluation (ΔPASI) and the absolute DLQI were used. After induction and during maintenance therapy, treatment can be continued if reduction in PASI is ≥75%. The treatment regimen should be modified if improvement of PASI is <50%. In a situation where the therapeutic response improved ≥50% but <75%, as assessed by PASI, therapy should be modified if the DLQI is >5 but can be continued if the DLQI is ≤5. This programme defines the severity of plaque psoriasis for the first time using a formal consensus of 19 European experts. In addition, treatment goals for moderate to severe disease were established. Implementation of treatment goals in the daily management of psoriasis will improve patient care and mitigate the problem of undertreatment. It is planned to evaluate the implementation of these treatment goals in a subsequent programme involving patients and physicians.
Figures
References
-
- Bergman MJ (2009) Assessing adequate treatment response in patients with rheumatoid arthritis. Clin Ther 31:1219–1231 - PubMed
-
- Cohen SB, Cohen MD, Cush JJ, et al. Unresolved issues in identifying, overcoming inadequate response in rheumatoid arthritis: weighing the evidence. J Rheumatol Suppl. 2008;81:4–30. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

