ROCK inhibition facilitates the generation of human-induced pluripotent stem cells in a defined, feeder-, and serum-free system
- PMID: 20858051
- PMCID: PMC2993021
- DOI: 10.1089/cell.2010.0051
ROCK inhibition facilitates the generation of human-induced pluripotent stem cells in a defined, feeder-, and serum-free system
Abstract
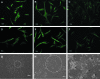
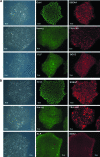
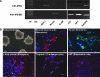
Human-induced pluripotent stem cells (iPSCs) generated from human adult somatic cells through reprogramming hold great promises for future regenerative medicine. However, exposure of human iPSCs to animal feeder and serum in the process of their generation and maintenance imposes risk of transmitting animal pathogens to human subjects, thus hindering the potential therapeutic applications. Here, we report the successful generation of human iPSCs in a feeder-independent culture system with defined factors. Two stable human iPSC lines were established from primary human dermal fibroblasts of two healthy volunteers. These human iPSCs expressed a panel of pluripotency markers including stage-specific embryonic antigen (SSEA)-4, tumor-rejection antigen (TRA)-1-60, TRA-1-81, and alkaline phosphatase, while maintaining normal karyotypes and the exogenous reprogramming factors being silenced. In addition, these human iPSCs can differentiate along lineages representative of the three embryonic germ layers upon formation of embryoid bodies, indicating their pluripotency. Furthermore, subcutaneous transplantation of these cells into immunodeficient mice resulted in teratoma formation in 6 to 8 weeks. Our findings are an important step toward generating patient-specific iPSCs in a more clinically compliant manner by eliminating the need of animal feeder cells and animal serum.
Figures

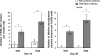



Similar articles
-
Gingival Fibroblasts as Autologous Feeders for Induced Pluripotent Stem Cells.J Dent Res. 2016 Jan;95(1):110-8. doi: 10.1177/0022034515611602. Epub 2015 Oct 14. J Dent Res. 2016. PMID: 26467419
-
Reduction of N-glycolylneuraminic acid in human induced pluripotent stem cells generated or cultured under feeder- and serum-free defined conditions.PLoS One. 2010 Nov 23;5(11):e14099. doi: 10.1371/journal.pone.0014099. PLoS One. 2010. PMID: 21124894 Free PMC article.
-
Mesenchymal stem cells as an appropriate feeder layer for prolonged in vitro culture of human induced pluripotent stem cells.Mol Biol Rep. 2013 Apr;40(4):3023-31. doi: 10.1007/s11033-012-2376-3. Epub 2013 Jan 3. Mol Biol Rep. 2013. PMID: 23283738
-
Induced pluripotent stem cells and hepatic differentiation.J Chin Med Assoc. 2013 Nov;76(11):599-605. doi: 10.1016/j.jcma.2013.07.007. Epub 2013 Aug 9. J Chin Med Assoc. 2013. PMID: 23933345 Review.
-
Mechanism and methods to induce pluripotency.Protein Cell. 2011 Oct;2(10):792-9. doi: 10.1007/s13238-011-1107-1. Epub 2011 Nov 6. Protein Cell. 2011. PMID: 22058034 Free PMC article. Review.
Cited by
-
An upregulation in the expression of vanilloid transient potential channels 2 enhances hypotonicity-induced cytosolic Ca²⁺ rise in human induced pluripotent stem cell model of Hutchinson-Gillford Progeria.PLoS One. 2014 Jan 27;9(1):e87273. doi: 10.1371/journal.pone.0087273. eCollection 2014. PLoS One. 2014. PMID: 24475260 Free PMC article.
-
Derivation, Expansion, and Motor Neuron Differentiation of Human-Induced Pluripotent Stem Cells with Non-Integrating Episomal Vectors and a Defined Xenogeneic-free Culture System.Mol Neurobiol. 2016 Apr;53(3):1589-1600. doi: 10.1007/s12035-014-9084-z. Epub 2015 Feb 10. Mol Neurobiol. 2016. PMID: 25663198
-
Modeling of Friedreich ataxia-related iron overloading cardiomyopathy using patient-specific-induced pluripotent stem cells.Pflugers Arch. 2014 Sep;466(9):1831-44. doi: 10.1007/s00424-013-1414-x. Epub 2013 Dec 11. Pflugers Arch. 2014. PMID: 24327207
-
Crosstalk between Substrates and Rho-Associated Kinase Inhibitors in Cryopreservation of Tissue-Engineered Constructs.Stem Cells Int. 2017;2017:1380304. doi: 10.1155/2017/1380304. Epub 2017 Oct 19. Stem Cells Int. 2017. PMID: 29201057 Free PMC article. Review.
-
Empagliflozin Ammeliorates High Glucose Induced-Cardiac Dysfuntion in Human iPSC-Derived Cardiomyocytes.Sci Rep. 2018 Oct 5;8(1):14872. doi: 10.1038/s41598-018-33293-2. Sci Rep. 2018. PMID: 30291295 Free PMC article.
References
-
- Amit M. Shariki C. Margulets V., et al. Feeder layer- and serum-free culture of human embryonic stem cells. Biol. Reprod. 2004;70:837–845. - PubMed
-
- Baharvand H. Salekdeh G.H. Taei A., et al. An efficient and easy-to-use cryopreservation protocol for human ES and iPS cells. Nat. Protoc. 2010;5:588–594. - PubMed
-
- Braam S.R. Nauw R. Ward-van Oostwaard D., et al. Inhibition of ROCK improves survival of human embryonic stem cell-derived cardiomyocytes after dissociation. Ann. N. Y. Acad. Sci. 2010;1188:52–57. - PubMed
-
- Chen M. Sun X. Jiang R., et al. Role of MEF feeder cells in direct reprogramming of mousetail-tip fibroblasts. Cell. Biol. Int. 2009;33:1268–1273. - PubMed
-
- Eiselleova L. Peterkova I. Neradil J., et al. Comparative study of mouse and human feeder cells for human embryonic stem cells. Int. J. Dev. Biol. 2008;52:353–363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
