Identification and characterization of a virus-specific continuous B-cell epitope on the PrM/M protein of Japanese Encephalitis Virus: potential application in the detection of antibodies to distinguish Japanese Encephalitis Virus infection from West Nile Virus and Dengue Virus infections
- PMID: 20858291
- PMCID: PMC2954857
- DOI: 10.1186/1743-422X-7-249
Identification and characterization of a virus-specific continuous B-cell epitope on the PrM/M protein of Japanese Encephalitis Virus: potential application in the detection of antibodies to distinguish Japanese Encephalitis Virus infection from West Nile Virus and Dengue Virus infections
Abstract
Background: Differential diagnose of Japanese encephalitis virus (JEV) infection from other flavivirus especially West Nile virus (WNV) and Dengue virus (DV) infection was greatly hindered for the serological cross-reactive. Virus specific epitopes could benefit for developing JEV specific antibodies detection methods. To identify the JEV specific epitopes, we fully mapped and characterized the continuous B-cell epitope of the PrM/M protein of JEV.
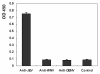
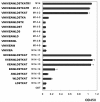
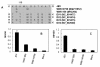
Results: To map the epitopes on the PrM/M protein, we designed a set of 20 partially overlapping fragments spanning the whole PrM, fused them with GST, and expressed them in an expression vector. Linear epitope M14 (105VNKKEAWLDSTKATRY120) was detected by enzyme-linked immunosorbent assay (ELISA). By removing amino acid residues individually from the carboxy and amino terminal of peptide M14, we confirmed that the minimal unit of the linear epitope of PrM/M was M14-13 (108KEAWLDSTKAT118). This epitope was highly conserved across different JEV strains. Moreover, this epitope did not cross-react with WNV-positive and DENV-positive sera.
Conclusion: Epitope M14-13 was a JEV specific lineal B-cell epitpe. The results may provide a useful basis for the development of epitope-based virus specific diagnostic clinical techniques.
Figures

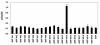


Similar articles
-
Comprehensive mapping of a novel NS1 epitope conserved in flaviviruses within the Japanese encephalitis virus serocomplex.Virus Res. 2014 Jun 24;185:103-9. doi: 10.1016/j.virusres.2014.03.001. Epub 2014 Mar 11. Virus Res. 2014. PMID: 24631788
-
Establishment of an Algorithm Using prM/E- and NS1-Specific IgM Antibody-Capture Enzyme-Linked Immunosorbent Assays in Diagnosis of Japanese Encephalitis Virus and West Nile Virus Infections in Humans.J Clin Microbiol. 2016 Feb;54(2):412-22. doi: 10.1128/JCM.02469-15. Epub 2015 Dec 9. J Clin Microbiol. 2016. PMID: 26659204 Free PMC article.
-
Identification of a conserved JEV serocomplex B-cell epitope by screening a phage-display peptide library with a mAb generated against West Nile virus capsid protein.Virol J. 2011 Mar 6;8:100. doi: 10.1186/1743-422X-8-100. Virol J. 2011. PMID: 21375771 Free PMC article.
-
Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses.Vaccine. 2010 Jan 8;28(3):632-49. doi: 10.1016/j.vaccine.2009.09.098. Epub 2009 Oct 4. Vaccine. 2010. PMID: 19808029 Review.
-
Serological cross-reactivity among common flaviviruses.Front Cell Infect Microbiol. 2022 Sep 15;12:975398. doi: 10.3389/fcimb.2022.975398. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36189346 Free PMC article. Review.
Cited by
-
Simultaneous detection and differentiation of dengue virus serotypes 1-4, Japanese encephalitis virus, and West Nile virus by a combined reverse-transcription loop-mediated isothermal amplification assay.Virol J. 2011 Jul 21;8:360. doi: 10.1186/1743-422X-8-360. Virol J. 2011. PMID: 21777455 Free PMC article.
-
Host Factor SPCS1 Regulates the Replication of Japanese Encephalitis Virus through Interactions with Transmembrane Domains of NS2B.J Virol. 2018 May 29;92(12):e00197-18. doi: 10.1128/JVI.00197-18. Print 2018 Jun 15. J Virol. 2018. PMID: 29593046 Free PMC article.
-
Comprehensive mapping infection-enhancing epitopes of dengue pr protein using polyclonal antibody against prM.Appl Microbiol Biotechnol. 2015 Jul;99(14):5917-27. doi: 10.1007/s00253-015-6538-9. Epub 2015 Mar 31. Appl Microbiol Biotechnol. 2015. PMID: 25822571 Free PMC article.
-
Production of recombinant nonstructural 1 protein in Escherichia coli for early detection of Japanese encephalitis virus infection.Microb Biotechnol. 2012 Sep;5(5):599-606. doi: 10.1111/j.1751-7915.2012.00344.x. Epub 2012 Mar 27. Microb Biotechnol. 2012. PMID: 22452851 Free PMC article.
-
Generation and characterization of a monoclonal antibody against prM protein of West Nile virus.Monoclon Antib Immunodiagn Immunother. 2014 Dec;33(6):438-43. doi: 10.1089/mab.2014.0047. Monoclon Antib Immunodiagn Immunother. 2014. PMID: 25514166 Free PMC article.
References
-
- Solomon T, Vaughn DW. Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections. Curr Top Microbiol Immunol. 2002;267:171–194. - PubMed
-
- Hanna JN, Ritchie SA, Phillips DA, Shield J, Bailey MC, Mackenzie JS, Poidinger M, McCall BJ, Mills PJ. An outbreak of Japanese encephalitis in the Torres Strait, Australia, 1995. Med J Aust. 1996;165:256–260. - PubMed
-
- van den Hurk AF, Nisbet DJ, Johansen CA, Foley PN, Ritchie SA, Mackenzie JS. Japanese encephalitis on Badu Island, Australia: the first isolation of Japanese encephalitis virus from Culex gelidus in the Australasian region and the role of mosquito host-feeding patterns in virus transmission cycles. Trans R Soc Trop Med Hyg. 2001;95:595–600. doi: 10.1016/S0035-9203(01)90090-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

