Fluorescence anisotropy reveals order and disorder of protein domains in the nuclear pore complex
- PMID: 20858414
- PMCID: PMC2941012
- DOI: 10.1016/j.bpj.2010.06.075
Fluorescence anisotropy reveals order and disorder of protein domains in the nuclear pore complex
Abstract
We present a new approach for studying individual protein domains within the nuclear pore complex (NPC) using fluorescence polarization microscopy. The NPC is a large macromolecular complex, the size and complexity of which presents experimental challenges. Using fluorescence anisotropy and exploiting the symmetry of the NPC and its organization in the nuclear envelope, we have resolved order and disorder of individual protein domains. Fluorescently tagging specific domains of individual nucleoporins revealed both rigid and flexible domains: the tips of the FG domains are disordered, whereas the NPC-anchored domains are ordered. Our technique allows the collection of structural information in vivo, providing the ability to probe the organization of protein domains within the NPC. This has particular relevance for the FG domain nucleoporins, which are crucial for nucleocytoplasmic transport.
Copyright © 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
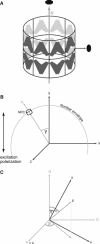
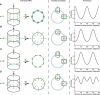


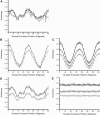
Similar articles
-
[Assembly and Disassembly of the Nuclear Pore Complex: A View from the Structural Side].Mol Biol (Mosk). 2023 Jul-Aug;57(4):573-586. Mol Biol (Mosk). 2023. PMID: 37528778 Russian.
-
Architecture of the cytoplasmic face of the nuclear pore.Science. 2022 Jun 10;376(6598):eabm9129. doi: 10.1126/science.abm9129. Epub 2022 Jun 10. Science. 2022. PMID: 35679405 Free PMC article.
-
Conserved spatial organization of FG domains in the nuclear pore complex.Biophys J. 2013 Jan 8;104(1):37-50. doi: 10.1016/j.bpj.2012.11.3823. Epub 2013 Jan 8. Biophys J. 2013. PMID: 23332057 Free PMC article.
-
Functional architecture of the nuclear pore complex.Annu Rev Biophys. 2012;41:557-84. doi: 10.1146/annurev-biophys-050511-102328. Annu Rev Biophys. 2012. PMID: 22577827 Review.
-
Flexible gates: dynamic topologies and functions for FG nucleoporins in nucleocytoplasmic transport.Eukaryot Cell. 2009 Dec;8(12):1814-27. doi: 10.1128/EC.00225-09. Epub 2009 Oct 2. Eukaryot Cell. 2009. PMID: 19801417 Free PMC article. Review.
Cited by
-
Illuminating cellular architecture and dynamics with fluorescence polarization microscopy.J Cell Sci. 2024 Oct 15;137(20):jcs261947. doi: 10.1242/jcs.261947. Epub 2024 Oct 14. J Cell Sci. 2024. PMID: 39404619 Review.
-
Nitric Oxide Activates β-Cell Glucokinase by Promoting Formation of the "Glucose-Activated" State.Biochemistry. 2018 Aug 28;57(34):5136-5144. doi: 10.1021/acs.biochem.8b00333. Epub 2018 Aug 10. Biochemistry. 2018. PMID: 30053375 Free PMC article.
-
Rapid and quantitative imaging of excitation polarized fluorescence reveals ordered septin dynamics in live yeast.Biophys J. 2011 Aug 17;101(4):985-94. doi: 10.1016/j.bpj.2011.07.008. Biophys J. 2011. PMID: 21843491 Free PMC article.
-
A Sensitized Emission Based Calibration of FRET Efficiency for Probing the Architecture of Macromolecular Machines.Cell Mol Bioeng. 2013;6(4):369-382. doi: 10.1007/s12195-013-0290-y. Epub 2013 Jul 11. Cell Mol Bioeng. 2013. PMID: 24319499 Free PMC article.
-
OOPS: Object-Oriented Polarization Software for analysis of fluorescence polarization microscopy images.PLoS Comput Biol. 2024 Aug 12;20(8):e1011723. doi: 10.1371/journal.pcbi.1011723. eCollection 2024 Aug. PLoS Comput Biol. 2024. PMID: 39133751 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

