Synthetic chloride-selective carbon nanotubes examined by using molecular and stochastic dynamics
- PMID: 20858417
- PMCID: PMC2941003
- DOI: 10.1016/j.bpj.2010.06.034
Synthetic chloride-selective carbon nanotubes examined by using molecular and stochastic dynamics
Abstract
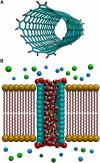
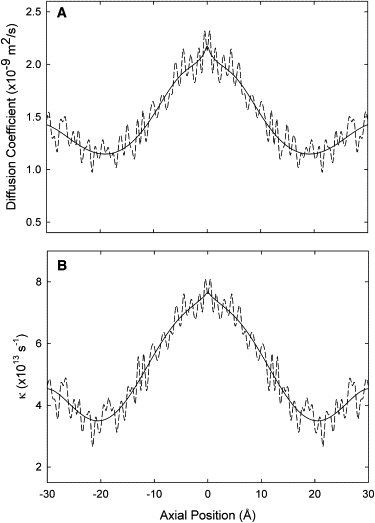
Synthetic channels, such as nanotubes, offer the possibility of ion-selective nanoscale pores which can broadly mimic the functions of various biological ion channels, and may one day be used as antimicrobial agents, or for treatment of cystic fibrosis. We have designed a carbon nanotube that is selectively permeable to anions. The virtual nanotubes are constructed from a hexagonal array of carbon atoms (graphene) rolled up to form a tubular structure, with an effective radius of 4.53 Å and length of 34 Å. The pore ends are terminated with polar carbonyl groups. The nanotube thus formed is embedded in a lipid bilayer and a reservoir containing ionic solutions is added at each end of the pore. The conductance properties of these synthetic channels are then examined with molecular and stochastic dynamics simulations. Profiles of the potential of mean force at 0 mM reveal that a cation moving across the pore encounters an insurmountable free energy barrier of ∼25 kT in height. In contrast, for anions, there are two energy wells of ∼12 kT near each end of the tube, separated by a central free energy barrier of 4 kT. The conductance of the pore, with symmetrical 500 mM solutions in the reservoirs, is 72 pS at 100 mV. The current saturates with an increasing ionic concentration, obeying a Michaelis-Menten relationship. The pore is normally occupied by two ions, and the rate-limiting step in conduction is the time taken for the resident ion near the exit gate to move out of the energy well.
Copyright © 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Synthetic cation-selective nanotube: permeant cations chaperoned by anions.J Chem Phys. 2011 Jan 28;134(4):045103. doi: 10.1063/1.3524310. J Chem Phys. 2011. PMID: 21280804
-
Designing biomimetic pores based on carbon nanotubes.Proc Natl Acad Sci U S A. 2012 May 1;109(18):6939-44. doi: 10.1073/pnas.1119326109. Epub 2012 Apr 16. Proc Natl Acad Sci U S A. 2012. PMID: 22509000 Free PMC article.
-
Stochastic transport through carbon nanotubes in lipid bilayers and live cell membranes.Nature. 2014 Oct 30;514(7524):612-5. doi: 10.1038/nature13817. Nature. 2014. PMID: 25355362
-
Computational modeling of transport in synthetic nanotubes.Nanomedicine. 2011 Dec;7(6):702-9. doi: 10.1016/j.nano.2011.02.011. Epub 2011 Mar 17. Nanomedicine. 2011. PMID: 21419868 Review.
-
Mechanical properties of lipid bilayers and regulation of mechanosensitive function: from biological to biomimetic channels.Channels (Austin). 2012 Jul-Aug;6(4):220-33. doi: 10.4161/chan.21085. Epub 2012 Jul 1. Channels (Austin). 2012. PMID: 22790280 Free PMC article. Review.
Cited by
-
Single-Walled Carbon Nanotubes: Mimics of Biological Ion Channels.Nano Lett. 2017 Feb 8;17(2):1204-1211. doi: 10.1021/acs.nanolett.6b04967. Epub 2017 Jan 19. Nano Lett. 2017. PMID: 28103039 Free PMC article.
-
Disruption of Model Cell Membranes by Carbon Nanotubes.Carbon N Y. 2013 Aug;60:67-75. doi: 10.1016/j.carbon.2013.03.057. Carbon N Y. 2013. PMID: 31007268 Free PMC article.
-
Computational design of a carbon nanotube fluorofullerene biosensor.Sensors (Basel). 2012 Oct 12;12(10):13720-35. doi: 10.3390/s121013720. Sensors (Basel). 2012. PMID: 23202018 Free PMC article.
-
Simulations on Simple Models of Connexin Hemichannels Indicate That Ca2+ Blocking Is Not a Pure Electrostatic Effect.Membranes (Basel). 2021 May 20;11(5):372. doi: 10.3390/membranes11050372. Membranes (Basel). 2021. PMID: 34065259 Free PMC article.
-
The very low number of calcium-induced permeability transition pores in the single mitochondrion.J Gen Physiol. 2020 Oct 5;152(10):e202012631. doi: 10.1085/jgp.202012631. J Gen Physiol. 2020. PMID: 32810269 Free PMC article.
References
-
- Rasaiah J.C., Garde S., Hummer G. Water in nonpolar confinement: from nanotubes to proteins and beyond. Annu. Rev. Phys. Chem. 2008;59:713–740. - PubMed
-
- Qu L., Lee K.M., Dai L. Carbon Nanotechnology. Elsevier; Amsterdam, The Netherlands: 2006. Functionalization and applications of carbon nanotubes.
-
- Hummer G., Rasaiah J.C., Noworyta J.P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature. 2001;414:188–190. - PubMed
-
- Waghe A., Rasaiah J.C., Hummer G. Filling and emptying kinetics of carbon nanotubes in water. J. Chem. Phys. 2002;117:10789–10795.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

