Cytochrome c-lipid interactions: new insights from resonance energy transfer
- PMID: 20858419
- PMCID: PMC2941011
- DOI: 10.1016/j.bpj.2010.06.017
Cytochrome c-lipid interactions: new insights from resonance energy transfer
Abstract
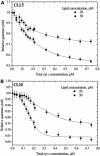
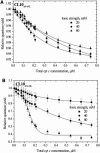
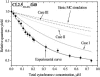
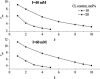
Resonance energy transfer (RET) from anthrylvinyl-labeled phosphatidylcholine (AV-PC) or cardiolipin (AV-CL) to cytochrome c (cyt c) heme moiety was employed to assess the molecular-level details of protein interactions with lipid bilayers composed of PC with 2.5 (CL2.5), 5 (CL5), 10 (CL10), or 20 (CL20) mol % CL under conditions of varying ionic strength and lipid/protein molar ratio. Monte Carlo analysis of multiple data sets revealed a subtle interplay between 1), exchange of the neutral and acidic lipid in the protein-lipid interaction zone; 2), CL transition into the extended conformation; and 3), formation of the hexagonal phase. The switch between these states was found to be controlled by CL content and salt concentration. At ionic strengths ≥ 40 mM, lipid bilayers with CL fraction not exceeding 5 mol % exhibited the tendency to transform from lamellar to hexagonal phase upon cyt c adsorption, whereas at higher contents of CL, transition into the extended conformation seems to become thermodynamically favorable. At lower ionic strengths, deviations from homogeneous lipid distributions were observed only for model membranes containing 2.5 mol % CL, suggesting the existence of a certain surface potential critical for assembly of lipid lateral domains in protein-lipid systems that may subsequently undergo morphological transformations depending on ambient conditions. These characteristics of cyt c-CL interaction are of great interest, not only from the viewpoint of regulating cyt c electron transfer and apoptotic propensities, but also to elucidate the general mechanisms by which membrane functional activities can be modulated by protein-lipid interactions.
Copyright © 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Förster Resonance Energy Transfer Study of Cytochrome c-Lipid Interactions.J Fluoresc. 2018 Jan;28(1):79-88. doi: 10.1007/s10895-017-2176-1. Epub 2017 Sep 6. J Fluoresc. 2018. PMID: 28879486
-
Cytochrome C interaction with cardiolipin/phosphatidylcholine model membranes: effect of cardiolipin protonation.Biophys J. 2006 Jun 1;90(11):4093-103. doi: 10.1529/biophysj.105.080150. Epub 2006 Mar 24. Biophys J. 2006. PMID: 16565064 Free PMC article.
-
Coverage-dependent changes of cytochrome c transverse location in phospholipid membranes revealed by FRET.Biochim Biophys Acta. 2005 Oct 1;1716(1):49-58. doi: 10.1016/j.bbamem.2005.09.002. Biochim Biophys Acta. 2005. PMID: 16183372
-
Structural transformations of cytochrome c upon interaction with cardiolipin.Chem Phys Lipids. 2014 Apr;179:57-63. doi: 10.1016/j.chemphyslip.2013.11.002. Epub 2013 Nov 16. Chem Phys Lipids. 2014. PMID: 24252639 Free PMC article. Review.
-
The physicochemical properties of cardiolipin bilayers and cardiolipin-containing lipid membranes.Biochim Biophys Acta. 2009 Oct;1788(10):2069-79. doi: 10.1016/j.bbamem.2009.03.014. Epub 2009 Mar 26. Biochim Biophys Acta. 2009. PMID: 19328771 Review.
Cited by
-
Versatility of non-native forms of human cytochrome c: pH and micellar concentration dependence.J Biol Inorg Chem. 2013 Jan;18(1):27-38. doi: 10.1007/s00775-012-0946-4. Epub 2012 Oct 16. J Biol Inorg Chem. 2013. PMID: 23070294
-
Nanodisc Films for Membrane Protein Studies by Neutron Reflection: Effect of the Protein Scaffold Choice.Langmuir. 2015 Aug 4;31(30):8386-91. doi: 10.1021/acs.langmuir.5b00936. Epub 2015 Jul 24. Langmuir. 2015. PMID: 26172514 Free PMC article.
-
The ionization properties of cardiolipin and its variants in model bilayers.Biochim Biophys Acta. 2016 Jun;1858(6):1362-72. doi: 10.1016/j.bbamem.2016.03.007. Epub 2016 Mar 7. Biochim Biophys Acta. 2016. PMID: 26965987 Free PMC article.
-
Mechanisms by Which Dietary Fatty Acids Regulate Mitochondrial Structure-Function in Health and Disease.Adv Nutr. 2018 May 1;9(3):247-262. doi: 10.1093/advances/nmy007. Adv Nutr. 2018. PMID: 29767698 Free PMC article. Review.
-
The mitochondria-targeted peptide SS-31 binds lipid bilayers and modulates surface electrostatics as a key component of its mechanism of action.J Biol Chem. 2020 May 22;295(21):7452-7469. doi: 10.1074/jbc.RA119.012094. Epub 2020 Apr 9. J Biol Chem. 2020. PMID: 32273339 Free PMC article.
References
-
- Pinheiro T.J.T., Cheng H., Roder H. Direct evidence for the cooperative unfolding of cytochrome c in lipid membranes from H-2H exchange kinetics. J. Mol. Biol. 2003;303:617–626. - PubMed
-
- Shidoji Y., Hayashi K., Yagi K. Loss of molecular interaction between cytochrome c and cardiolipin due to lipid peroxidation. Biochem. Biophys. Res. Commun. 1999;264:343–347. - PubMed
-
- Schug Z.T., Gottlieb E. Cardiolipin acts as a mitochondrial signalling platform to launch apoptosis. Biochim. Biophys. Acta. 2009;1788:2022–2031. - PubMed
-
- Ostrander D.B., Sparagna G.C., Dowhan W. Decreased cardiolipin synthesis corresponds with cytochrome c release in palmitate-induced cardiomyocyte apoptosis. J. Biol. Chem. 2001;276:38061–38067. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

