Structural role of the conserved cysteines in the dimerization of the viral transmembrane oncoprotein E5
- PMID: 20858420
- PMCID: PMC2941001
- DOI: 10.1016/j.bpj.2010.06.073
Structural role of the conserved cysteines in the dimerization of the viral transmembrane oncoprotein E5
Abstract
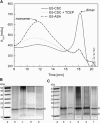
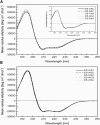
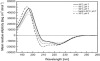
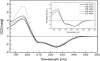
The E5 oncoprotein is the major transforming protein of bovine papillomavirus type 1. This 44-residue transmembrane protein can interact with the platelet-derived growth factor receptor β, leading to ligand-independent activation and cell transformation. For productive interaction, E5 needs to dimerize via a C-terminal pair of cysteines, though a recent study suggested that its truncated transmembrane segment can dimerize on its own. To analyze the structure of the full protein in a membrane environment and elucidate the role of the Cys-Ser-Cys motif, we produced recombinantly the wild-type protein and four cysteine mutants. Comparison by circular dichroism in detergent micelles and lipid vesicular dispersion and by NMR in trifluoroethanol demonstrates that the absence of one or both cysteines does not influence the highly α-helical secondary structure, nor does it impair the ability of E5 to dimerize, observations that are further supported by sodium dodecylsulfate polyacrylamide gel electrophoresis. We also observed assemblies of higher order. Oriented circular dichroism in lipid bilayers shows that E5 is aligned as a transmembrane helix with a slight tilt angle, and that this membrane alignment is also independent of any cysteines. We conclude that the Cys-containing motif represents a disordered region of the protein that serves as an extra covalent connection for stabilization.
Copyright © 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Structural characterization of a C-terminally truncated E5 oncoprotein from papillomavirus in lipid bilayers.Biol Chem. 2014 Dec;395(12):1443-52. doi: 10.1515/hsz-2014-0222. Biol Chem. 2014. PMID: 25324446
-
Cellular transformation by a transmembrane peptide: structural requirements for the bovine papillomavirus E5 oncoprotein.Proc Natl Acad Sci U S A. 1994 May 24;91(11):4634-8. doi: 10.1073/pnas.91.11.4634. Proc Natl Acad Sci U S A. 1994. PMID: 8197111 Free PMC article.
-
Towards a structural understanding of the smallest known oncoprotein: investigation of the bovine papillomavirus E5 protein using solution-state NMR.Biochim Biophys Acta. 2011 Jun;1808(6):1493-501. doi: 10.1016/j.bbamem.2010.11.004. Epub 2010 Nov 10. Biochim Biophys Acta. 2011. PMID: 21073859
-
The bovine papillomavirus E5 protein and the PDGF beta receptor: it takes two to tango.Virology. 2009 Feb 20;384(2):345-51. doi: 10.1016/j.virol.2008.09.033. Epub 2008 Nov 6. Virology. 2009. PMID: 18990418 Free PMC article. Review.
-
The platelet-derived growth factor beta receptor as a target of the bovine papillomavirus E5 protein.Cytokine Growth Factor Rev. 2000 Dec;11(4):283-93. doi: 10.1016/s1359-6101(00)00012-5. Cytokine Growth Factor Rev. 2000. PMID: 10959076 Review.
Cited by
-
Structure of the membrane anchor of pestivirus glycoprotein E(rns), a long tilted amphipathic helix.PLoS Pathog. 2014 Feb 27;10(2):e1003973. doi: 10.1371/journal.ppat.1003973. eCollection 2014 Feb. PLoS Pathog. 2014. PMID: 24586172 Free PMC article.
-
Hydrophobic Mismatch Drives the Interaction of E5 with the Transmembrane Segment of PDGF Receptor.Biophys J. 2015 Aug 18;109(4):737-49. doi: 10.1016/j.bpj.2015.07.022. Biophys J. 2015. PMID: 26287626 Free PMC article.
-
Two transmembrane dimers of the bovine papillomavirus E5 oncoprotein clamp the PDGF β receptor in an active dimeric conformation.Proc Natl Acad Sci U S A. 2017 Aug 29;114(35):E7262-E7271. doi: 10.1073/pnas.1705622114. Epub 2017 Aug 14. Proc Natl Acad Sci U S A. 2017. PMID: 28808001 Free PMC article.
-
Hydrophobic matching controls the tilt and stability of the dimeric platelet-derived growth factor receptor (PDGFR) β transmembrane segment.J Biol Chem. 2012 Jul 27;287(31):26178-86. doi: 10.1074/jbc.M111.325555. Epub 2012 May 22. J Biol Chem. 2012. PMID: 22619173 Free PMC article.
-
A single amino acid substitution converts a transmembrane protein activator of the platelet-derived growth factor β receptor into an inhibitor.J Biol Chem. 2013 Sep 20;288(38):27273-27286. doi: 10.1074/jbc.M113.470054. Epub 2013 Aug 1. J Biol Chem. 2013. PMID: 23908351 Free PMC article.
References
-
- Yarden Y., Schlessinger J. Epidermal growth factor induces rapid, reversible aggregation of the purified epidermal growth factor receptor. Biochemistry. 1987;26:1443–1451. - PubMed
-
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. - PubMed
-
- Yarden Y., Schlessinger J. Self-phosphorylation of epidermal growth factor receptor: evidence for a model of intermolecular allosteric activation. Biochemistry. 1987;26:1434–1442. - PubMed
-
- Jorissen R.N., Walker F., Burgess A.W. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp. Cell Res. 2003;284:31–53. - PubMed
-
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

