Anesthetic binding in a pentameric ligand-gated ion channel: GLIC
- PMID: 20858424
- PMCID: PMC2941008
- DOI: 10.1016/j.bpj.2010.07.023
Anesthetic binding in a pentameric ligand-gated ion channel: GLIC
Abstract
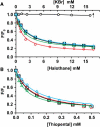
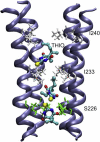
Cys-loop receptors are molecular targets of general anesthetics, but the knowledge of anesthetic binding to these proteins remains limited. Here we investigate anesthetic binding to the bacterial Gloeobacter violaceus pentameric ligand-gated ion channel (GLIC), a structural homolog of cys-loop receptors, using an experimental and computational hybrid approach. Tryptophan fluorescence quenching experiments showed halothane and thiopental binding at three tryptophan-associated sites in the extracellular (EC) domain, transmembrane (TM) domain, and EC-TM interface of GLIC. An additional binding site at the EC-TM interface was predicted by docking analysis and validated by quenching experiments on the N200W GLIC mutant. The binding affinities (K(D)) of 2.3 ± 0.1 mM and 0.10 ± 0.01 mM were derived from the fluorescence quenching data of halothane and thiopental, respectively. Docking these anesthetics to the original GLIC crystal structure and the structures relaxed by molecular dynamics simulations revealed intrasubunit sites for most halothane binding and intersubunit sites for thiopental binding. Tryptophans were within reach of both intra- and intersubunit binding sites. Multiple molecular dynamics simulations on GLIC in the presence of halothane at different sites suggested that anesthetic binding at the EC-TM interface disrupted the critical interactions for channel gating, altered motion of the TM23 linker, and destabilized the open-channel conformation that can lead to inhibition of GLIC channel current. The study has not only provided insights into anesthetic binding in GLIC, but also demonstrated a successful fusion of experiments and computations for understanding anesthetic actions in complex proteins.
Copyright © 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Propofol binding to the resting state of the gloeobacter violaceus ligand-gated ion channel (GLIC) induces structural changes in the inter- and intrasubunit transmembrane domain (TMD) cavities.J Biol Chem. 2013 Jun 14;288(24):17420-31. doi: 10.1074/jbc.M113.464040. Epub 2013 May 2. J Biol Chem. 2013. PMID: 23640880 Free PMC article.
-
Isoflurane alters the structure and dynamics of GLIC.Biophys J. 2011 Oct 19;101(8):1905-12. doi: 10.1016/j.bpj.2011.09.026. Biophys J. 2011. PMID: 22004744 Free PMC article.
-
Ketamine Inhibition of the Pentameric Ligand-Gated Ion Channel GLIC.Biophys J. 2017 Aug 8;113(3):605-612. doi: 10.1016/j.bpj.2017.06.041. Biophys J. 2017. PMID: 28793215 Free PMC article.
-
From hopanoids to cholesterol: Molecular clocks of pentameric ligand-gated ion channels.Prog Lipid Res. 2016 Jul;63:1-13. doi: 10.1016/j.plipres.2016.03.003. Epub 2016 Apr 12. Prog Lipid Res. 2016. PMID: 27084463 Review.
-
Computational studies on the interactions of inhalational anesthetics with proteins.Acc Chem Res. 2010 Jan 19;43(1):103-10. doi: 10.1021/ar900149j. Acc Chem Res. 2010. PMID: 19788306 Review.
Cited by
-
Inhibition versus potentiation of ligand-gated ion channels can be altered by a single mutation that moves ligands between intra- and intersubunit sites.Structure. 2013 Aug 6;21(8):1307-16. doi: 10.1016/j.str.2013.06.018. Epub 2013 Jul 25. Structure. 2013. PMID: 23891290 Free PMC article.
-
Propofol inhibits the voltage-gated sodium channel NaChBac at multiple sites.J Gen Physiol. 2018 Sep 3;150(9):1317-1331. doi: 10.1085/jgp.201811993. Epub 2018 Jul 17. J Gen Physiol. 2018. PMID: 30018039 Free PMC article.
-
Asymmetric ligand binding facilitates conformational transitions in pentameric ligand-gated ion channels.J Am Chem Soc. 2013 Feb 13;135(6):2172-80. doi: 10.1021/ja307275v. Epub 2013 Feb 4. J Am Chem Soc. 2013. PMID: 23339564 Free PMC article.
-
Common Internal Allosteric Network Links Anesthetic Binding Sites in a Pentameric Ligand-Gated Ion Channel.PLoS One. 2016 Jul 12;11(7):e0158795. doi: 10.1371/journal.pone.0158795. eCollection 2016. PLoS One. 2016. PMID: 27403526 Free PMC article.
-
Molecular dynamics and brownian dynamics investigation of ion permeation and anesthetic halothane effects on a proton-gated ion channel.J Am Chem Soc. 2010 Nov 24;132(46):16442-9. doi: 10.1021/ja105001a. Epub 2010 Oct 27. J Am Chem Soc. 2010. PMID: 20979415 Free PMC article.
References
-
- Franks N.P., Lieb W.R. Molecular and cellular mechanisms of general anesthesia. Nature. 1994;367:607–614. - PubMed
-
- Hemmings H.C., Jr., Akabas M.H., Harrison N.L. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol. Sci. 2005;26:503–510. - PubMed
-
- Chiara D.C., Dangott L.J., Cohen J.B. Identification of nicotinic acetylcholine receptor amino acids photolabeled by the volatile anesthetic halothane. Biochemistry. 2003;42:13457–13467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

