Kinesin recycling in stationary membrane tubes
- PMID: 20858428
- PMCID: PMC2940996
- DOI: 10.1016/j.bpj.2010.06.071
Kinesin recycling in stationary membrane tubes
Abstract
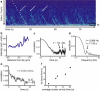
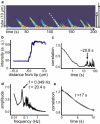
Collections of motors dynamically organize to extract membrane tubes. These tubes grow but often pause or change direction as they traverse an underlying microtubule (MT) network. In vitro, membrane tubes also stall: they stop growing in length despite a large group of motors available at the tip to pull them forward. In these stationary membrane tubes in vitro, we find that clusters of processive kinesin motors form and reach the tip of the tube at regular time intervals. The average times between cluster arrivals depends on the time over which motors depart from the tip, suggesting that motors are recycled toward the tip. Numerical simulations of the motor dynamics in the membrane tube and on the MTs show that the presence of cooperative binding between motors quantitatively accounts for the clustering observed experimentally. Cooperative binding along the length of the MT and a nucleation point at a distance behind the tip define the recycling period. Based on comparison of the numerical results and experimental data, we estimate a cooperative binding probability and concentration regime where the recycling phenomenon occurs.
Copyright © 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Bidirectional membrane tube dynamics driven by nonprocessive motors.Proc Natl Acad Sci U S A. 2008 Jun 10;105(23):7993-7. doi: 10.1073/pnas.0709677105. Epub 2008 Mar 10. Proc Natl Acad Sci U S A. 2008. PMID: 18332438 Free PMC article.
-
Competition between kinesin-1 and myosin-V defines Drosophila posterior determination.Elife. 2020 Feb 14;9:e54216. doi: 10.7554/eLife.54216. Elife. 2020. PMID: 32057294 Free PMC article.
-
Dynamic kinesin-1 clustering on microtubules due to mutually attractive interactions.Phys Biol. 2008 Nov 24;5(4):046004. doi: 10.1088/1478-3975/5/4/046004. Phys Biol. 2008. PMID: 19029597
-
Insights into the process of EB1-dependent tip-tracking of kinesin-14 Ncd. The role of the microtubule.Eur J Cell Biol. 2016 Dec;95(12):521-530. doi: 10.1016/j.ejcb.2016.08.004. Epub 2016 Aug 31. Eur J Cell Biol. 2016. PMID: 27608966
-
Mechanism of membrane nanotube formation by molecular motors.Biochim Biophys Acta. 2010 Jul;1798(7):1418-26. doi: 10.1016/j.bbamem.2009.11.012. Epub 2009 Nov 27. Biochim Biophys Acta. 2010. PMID: 19948146 Review.
Cited by
-
Opposing Kinesin and Myosin-I Motors Drive Membrane Deformation and Tubulation along Engineered Cytoskeletal Networks.Curr Biol. 2018 Jan 22;28(2):236-248.e5. doi: 10.1016/j.cub.2017.12.007. Epub 2018 Jan 11. Curr Biol. 2018. PMID: 29337076 Free PMC article.
-
Single molecule fluorescence detection and tracking in mammalian cells: the state-of-the-art and future perspectives.Int J Mol Sci. 2012 Nov 13;13(11):14742-65. doi: 10.3390/ijms131114742. Int J Mol Sci. 2012. PMID: 23203092 Free PMC article. Review.
References
-
- Howard J. Sinauer; Sunderland, MA: 2001. Mechanics of Motor Proteins and the Cytoskeleton.
-
- Hill D.B., Plaza M.J., Holzwarth G. Fast vesicle transport in PC12 neurites: velocities and forces. Eur. Biophys. J. 2004;33:623–632. - PubMed
-
- Kural C., Kim H., Selvin P.R. Kinesin and dynein move a peroxisome in vivo: a tug-of-war or coordinated movement? Science. 2005;308:1469–1472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

