Second harmonic generation microscopy probes different states of motor protein interaction in myofibrils
- PMID: 20858429
- PMCID: PMC2941014
- DOI: 10.1016/j.bpj.2010.07.005
Second harmonic generation microscopy probes different states of motor protein interaction in myofibrils
Abstract
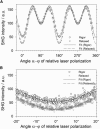
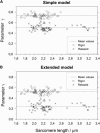
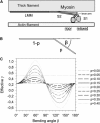
The second harmonic generation (SHG) signal intensity sourced from skeletal muscle myosin II strongly depends on the polarization of the incident laser beam relative to the muscle fiber axis. This dependence is related to the second-order susceptibility χ((2)), which can be described by a single component ratio γ under generally assumed symmetries. We precisely extracted γ from SHG polarization dependence curves with an extended focal field model. In murine myofibrillar preparations, we have found two distinct polarization dependencies: With the actomyosin system in the rigor state, γ(rig) has a mean value of γ(rig) = 0.52 (SD = 0.04, n = 55); in a relaxed state where myosin is not bound to actin, γ(rel) has a mean value of γ(rel) = 0.24 (SD = 0.07, n = 70). We observed a similar value in an activated state where the myosin power stroke was pharmacologically inhibited using N-benzyl-p-toluene sulfonamide. In summary, different actomyosin states can be visualized noninvasively with SHG microscopy. Specifically, SHG even allows us to distinguish different actin-bound states of myosin II using γ as a parameter.
Copyright © 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Rotational dynamics of actin-bound intermediates of the myosin adenosine triphosphatase cycle in myofibrils.Biophys J. 1994 Jul;67(1):250-61. doi: 10.1016/S0006-3495(94)80476-X. Biophys J. 1994. PMID: 7918993 Free PMC article.
-
Characterization of the myosin-based source for second-harmonic generation from muscle sarcomeres.Biophys J. 2006 Jan 15;90(2):693-703. doi: 10.1529/biophysj.105.071555. Epub 2005 Oct 28. Biophys J. 2006. PMID: 16258040 Free PMC article.
-
Second harmonic generation polarization properties of myofilaments.J Biomed Opt. 2014 May;19(5):056005. doi: 10.1117/1.JBO.19.5.056005. J Biomed Opt. 2014. PMID: 24805809
-
[Optical Second-harmonic Observation of Stimulated Sacran Aggregates].Yakugaku Zasshi. 2019;139(3):351-362. doi: 10.1248/yakushi.18-00177-1. Yakugaku Zasshi. 2019. PMID: 30828011 Review. Japanese.
-
Molecular mechanism of actin-myosin motor in muscle.Biochemistry (Mosc). 2011 Dec;76(13):1484-506. doi: 10.1134/S0006297911130086. Biochemistry (Mosc). 2011. PMID: 22339600 Review.
Cited by
-
Changes in the crystallographic structures of cardiac myosin filaments detected by polarization-dependent second harmonic generation microscopy.Biomed Opt Express. 2019 Jun 7;10(7):3183-3195. doi: 10.1364/BOE.10.003183. eCollection 2019 Jul 1. Biomed Opt Express. 2019. PMID: 31360597 Free PMC article.
-
Second Harmonic Generation Morphometry of Muscle Cytoarchitecture in Living Cells.Methods Mol Biol. 2023;2644:267-285. doi: 10.1007/978-1-0716-3052-5_17. Methods Mol Biol. 2023. PMID: 37142928
-
Estimation of crossbridge-state during cardiomyocyte beating using second harmonic generation.Life Sci Alliance. 2023 May 26;6(7):e202302070. doi: 10.26508/lsa.202302070. Print 2023 Jul. Life Sci Alliance. 2023. PMID: 37236659 Free PMC article.
-
Visualization of mouse neuronal ganglia infected by Herpes Simplex Virus 1 (HSV-1) using multimodal non-linear optical microscopy.PLoS One. 2014 Aug 18;9(8):e105103. doi: 10.1371/journal.pone.0105103. eCollection 2014. PLoS One. 2014. PMID: 25133579 Free PMC article.
-
Polarization-resolved second harmonic microscopy of skeletal muscle in sepsis.Biomed Opt Express. 2018 Nov 19;9(12):6350-6358. doi: 10.1364/BOE.9.006350. eCollection 2018 Dec 1. Biomed Opt Express. 2018. PMID: 31065433 Free PMC article.
References
-
- Franken P.A., Hill A.E., Weinreich G. Generation of optical harmonics. Phys. Rev. Lett. 1961;7:118–119.
-
- Roth S., Freund I. Second harmonic generation in collagen. J. Chem. Phys. 1979;70:1637–1643.
-
- Both M., Vogel M., Uttenweiler D. Second harmonic imaging of intrinsic signals in muscle fibers in situ. J. Biomed. Opt. 2004;9:882–892. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

